|
Ranunculin
Ranunculin is a glycoside found in many members of the Ranunculaceae, buttercup family, including species of Helleborus, Anemone, Clematis and most commonly Ranunculus. Glycosides are common in plants, where they serve as defense mechanisms against herbivores and microorganisms. When plant cell wall structures are damaged, glycosidase enzymes hydrolyze the inactive glycoside into its components- a sugar and practically any other molecule, which is called the aglycone. Ranunculin is a glucoside, which indicates that glucose is the specific sugar attached its aglycone protoanemonin. Toxicity Ranunculin is very stable in acidic medium and fresh plant tissues, but in alkaline solution or damaged plant cells is hydrolyzed into the unstable toxin protoanemonin and glucose. Protoanemonin release References {{reflist, refs= {{cite journal , last1=Bai , first1=Yili , last2=Benn , first2=Michael , last3=Majak , first3=Walter , last4=McDiamid , first4=Ruth , title=Extraction and HPLC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helleborus
Commonly known as hellebores (), the Eurasian genus ''Helleborus'' consists of approximately 20 species of herbaceous or evergreen perennial flowering plants in the family Ranunculaceae, within which it gave its name to the tribe of Helleboreae. Many hellebore species are poisonous. Despite common names such as ''winter rose'', ''Christmas rose'', and ''Lenten rose'', hellebores are not closely related to the rose family ( Rosaceae). Etymology The common name "hellebore" is first attested in 1300s; it originates, via Old French and Latin">-4; we might wonder whether there's a point at which it's appropriate to talk of the beginnings of French, that is, when it wa ... and Latin, ultimately from . Although traditionally translated as "plant eaten by fawns", this could be folk etymology and, according to Beekes, really a Pre-Greek word. It is false friend, ''not'' related to the word "hell", despite the toxic nature of this plant. In Anglo-Saxon England, the Anglo-Latin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protoanemonin
Protoanemonin (sometimes called anemonol or ranunculol) is a toxin whose glyosidic precursor ranunculin is found in many plants of the buttercup family (Ranunculaceae). When the plant is wounded or macerated, ranunculin is enzymatically broken down into glucose and protoanemonin. This toxin's ability to inhibit both gram positive and gram negative bacteria is linked to the presence of a 5-membered lactone ring with a highly reactive double bond system. Biological pathway Toxicity Protoamenonin has vesicant properties, which cause rashes or blistering upon contact with the skin or mucosa. Ingesting large amounts of the toxin despite its bitter taste can cause nausea, vomiting, dizziness, spasms, acute hepatitis, jaundice, or paralysis in animals and humans. Safety At room temperature, protoanemonin spontaneously dimerizes into the potentially therapeutic compound anemonin, which can then be hydrolyzed into a dicarboxylic acid. As such, plants containing glycosidic precursors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
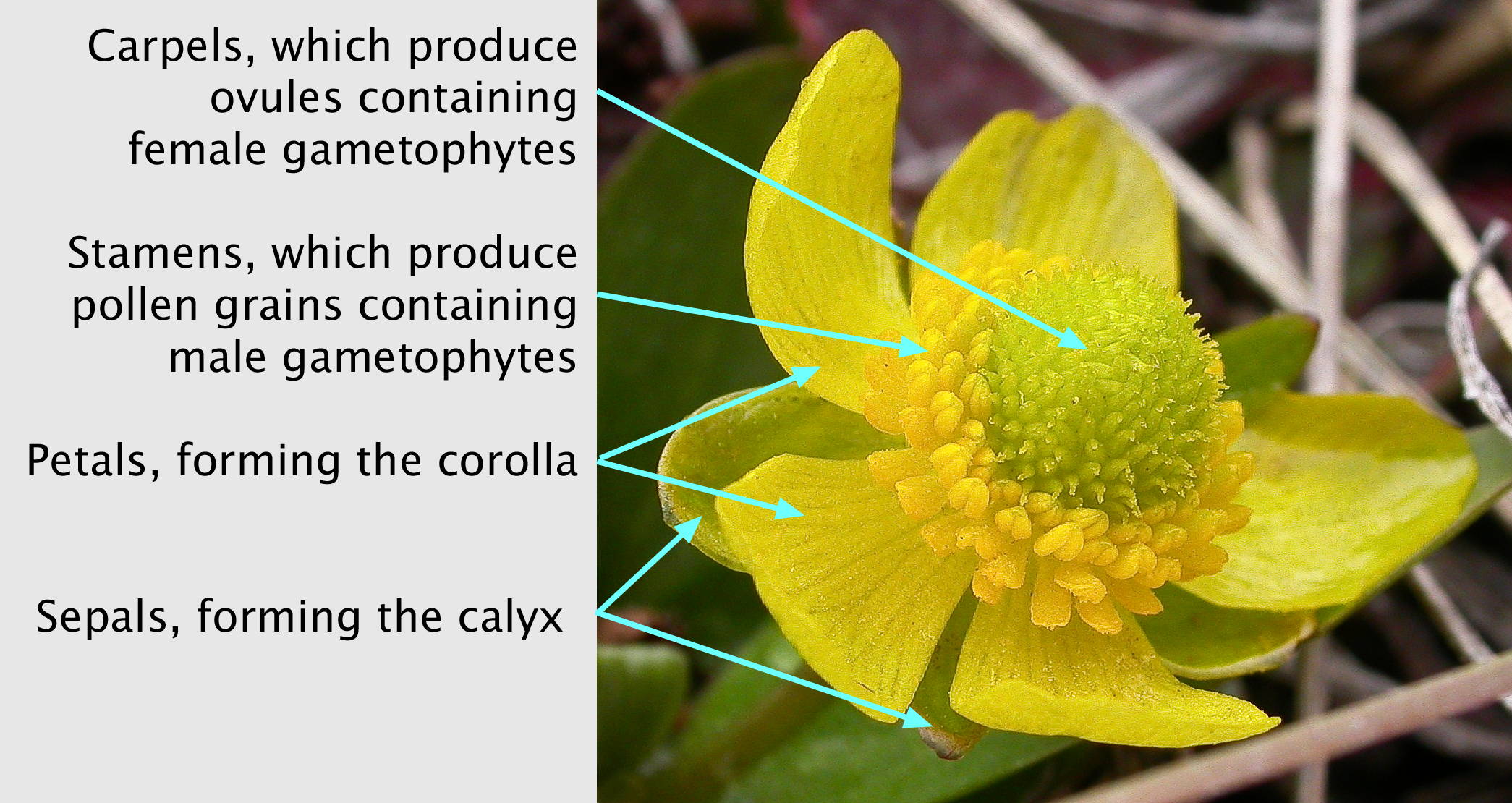
Ranunculus
''Ranunculus'' is a large genus of about 1750 species of flowering plants in the family Ranunculaceae. Members of the genus are known as buttercups, spearworts and water crowfoots. The genus is distributed worldwide, primarily in temperate and montane regions. The familiar and widespread buttercup of gardens throughout Northern Europe (and introduced elsewhere) is the creeping buttercup '' Ranunculus repens'', which has extremely tough and tenacious roots. Two other species are also widespread, the bulbous buttercup '' Ranunculus bulbosus'' and the much taller meadow buttercup '' Ranunculus acris''. In ornamental gardens, all three are often regarded as weeds. Buttercups usually flower in the spring, but flowers may be found throughout the summer, especially where the plants are growing as opportunistic colonizers, as in the case of garden weeds. The water crowfoots (''Ranunculus'' subgenus ''Batrachium''), which grow in still or running water, are sometimes treated in a sep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ranunculin Skeletal
Ranunculin is a glycoside found in many members of the buttercup family, including species of Helleborus, Anemone, Clematis and most commonly Ranunculus. Glycosides are common in plants, where they serve as defense mechanisms against herbivores and microorganisms. When plant cell wall structures are damaged, glycosidase enzymes hydrolyze the inactive glycoside into its components- a sugar and practically any other molecule, which is called the aglycone. Ranunculin is a glucoside, which indicates that glucose is the specific sugar attached its aglycone protoanemonin. Toxicity Ranunculin is very stable in acidic medium and fresh plant tissues, but in alkaline solution or damaged plant cells is hydrolyzed into the unstable toxin protoanemonin Protoanemonin (sometimes called anemonol or ranunculol) is a toxin whose glyosidic precursor ranunculin is found in many plants of the buttercup family (Ranunculaceae). When the plant is wounded or macerated, ranunculin is enzymatically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzymatic, enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an ''O-glycoside''), N- (a ''glycosylamine''), S-(a ''thioglycoside''), or C- (a ''C-glycoside'') glycosidic bond. Accord ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. It is used by plants to make cellulose, the most abundant carbohydrate in the world, for use in cell walls, and by all living Organism, organisms to make adenosine triphosphate (ATP), which is used by the cell as energy. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as amylose and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form is -glucose, while its Stereoisomerism, stereoisomer L-glucose, -glucose is produced synthetically in comparatively small amounts and is less biologicall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucosides
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is Hydrolysis, hydrolysed by purely chemical means, or decomposed by fermentation (biochemistry), fermentation or enzymes. The name was originally given to plant products of this nature, in which the other part of the molecule was, in the greater number of cases, an aromatic aldehydic or natural phenol, phenolic compound (exceptions are sinigrin, Jinigrin and Jalapin or Scammonin I, Scammonin). It has now been extended to include synthetic ethers, such as those obtained by acting on ethanol, alcoholic glucose solutions with hydrochloric acid, and also the polysaccharoses, e.g. cane sugar, which appear to be ethers also. Although glucose is the most common sugar present in glucosides, many are known which yield rhamnose or iso-dulcite; these may be termed pentosides. Much attention has been given to the non-sugar parts (agly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a base, and they are still among the most common bases. Etymology The word ''alkali'' is derived from Arabic ''al qalīy'' (or ''alkali''), meaning (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (''slaked lime''), a far more strongly basic substance known as ''caustic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acidic
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Arrhenius acids. Brønsted and Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and react with bases and certain metals (like calcium) to form salts. The word ''acid'' is derived from the Latin , meaning 'sour'. An aqueous solution of an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aglycone
An aglycone (aglycon or genin) is the chemical compound remaining after the glycosyl group on a glycoside is replaced by a hydrogen atom. For example, the aglycone of a cardiac glycoside would be a steroid A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes t ... molecule. Detection A way to identify aglycone is proposed to extract it from Agave spp. by using H-NMR and Heteronuclear multiple bond correlation (HMBC) experiments. The HMBC experiment can be combined with other techniques such as mass spectrometry to further examine the structure and the function of aglycone. Samples of glycones and glycosides from limonoids can be simultaneously quantified through a high performance liquid chromatography (HPLC) method, where a binary solvent system and a diode array detector separate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucoside
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes. The name was originally given to plant products of this nature, in which the other part of the molecule was, in the greater number of cases, an aromatic aldehydic or phenolic compound (exceptions are Jinigrin and Jalapin or Scammonin). It has now been extended to include synthetic ethers, such as those obtained by acting on alcoholic glucose solutions with hydrochloric acid, and also the polysaccharoses, e.g. cane sugar, which appear to be ethers also. Although glucose is the most common sugar present in glucosides, many are known which yield rhamnose or iso-dulcite; these may be termed pentosides. Much attention has been given to the non-sugar parts (aglycone) of the molecules; the constitutions of many have been determined, and the comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |