|
Radical SAM Enzymes
Radical SAM enzymes belong to a superfamily of enzymes that use an iron-sulfur cluster ( 4Fe-4S) to reductively cleave ''S''-adenosyl-L-methionine (SAM) to generate a radical, usually a 5′- deoxyadenosyl radical (5'-dAdo), as a critical intermediate. These enzymes utilize this radical intermediate to perform diverse transformations, often to functionalize unactivated C-H bonds. Radical SAM enzymes are involved in cofactor biosynthesis, enzyme activation, peptide modification, post-transcriptional and post-translational modifications, metalloprotein cluster formation, tRNA modification, lipid metabolism, biosynthesis of antibiotics and natural products etc. The vast majority of known radical SAM enzymes belong to the radical SAM superfamily, and have a cysteine-rich motif that matches or resembles CxxxCxxC. Radical SAM enzymes comprise the largest superfamily of metal-containing enzymes. History and mechanism As of 2001, 645 unique radical SAM enzymes have been identified fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyltransferase
Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossmann fold for binding ''S''-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltransferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salmonella Enterica Subsp
''Salmonella'' is a genus of rod-shaped, (bacillus) Gram-negative bacteria of the family Enterobacteriaceae. The two known species of ''Salmonella'' are '' Salmonella enterica'' and '' Salmonella bongori''. ''S. enterica'' is the type species and is further divided into six subspecies that include over 2,650 serotypes. ''Salmonella'' was named after Daniel Elmer Salmon (1850–1914), an American veterinary surgeon. ''Salmonella'' species are non-spore-forming, predominantly motile enterobacteria with cell diameters between about 0.7 and 1.5 μm, lengths from 2 to 5 μm, and peritrichous flagella (all around the cell body, allowing them to move). They are chemotrophs, obtaining their energy from oxidation and reduction reactions, using organic sources. They are also facultative anaerobes, capable of generating adenosine triphosphate with oxygen ("aerobically") when it is available, or using other electron acceptors or fermentation ("anaerobically") w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiolation
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic, cabbage or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the smell of natural gas is due to the smell of the thiol used as the odorant. Nomenclature Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticodons
Transfer ribonucleic acid (tRNA), formerly referred to as soluble ribonucleic acid (sRNA), is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes). In a cell, it provides the physical link between the genetic code in messenger RNA (mRNA) and the amino acid sequence of proteins, carrying the correct sequence of amino acids to be combined by the protein-synthesizing machinery, the ribosome. Each three-nucleotide codon in mRNA is complemented by a three-nucleotide anticodon in tRNA. As such, tRNAs are a necessary component of translation, the biological synthesis of new proteins in accordance with the genetic code. Overview The process of translation starts with the information stored in the nucleotide sequence of DNA. This is first transformed into mRNA, then tRNA specifies which three-nucleotide codon from the genetic code corresponds to which amino acid. Each mRNA codon is recognized by a particular type of tRNA, which docks to it along a t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylthiotransferase
Methylthiotransferases are enzymes of the Radical SAM, radical S-adenosyl methionine (radical SAM) superfamily. These enzymes catalyze the addition of a methylthio group to various biochemical compounds including tRNA and proteins. Methylthiotransferases are classified into one of four classes based on their substrates and mechanisms. All methylthiotransferases have been shown to contain two Fe-S clusters, one canonical cluster and one auxiliary cluster, that both function in the addition of the methylthio group to the substrate. Overview Methylthiotransferases, also known as MTTases, are a subset of the radical SAM enzyme superfamily. These enzymes catalyze the addition of a methylthio group to either a protein or tRNA substrate. Radical S-adenosylmethionine enzymes, otherwise known as radical SAM enzymes, are metalloproteins that cleave S-adenosyl-L-methionine into L-methionine and a 5'-deoxyadenosyl 5'-radical (5'-dA). 5'-dA is an intermediate in the reactions catalyzed by radic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives - cyclopropanes - are of commercial or biological significance. Cyclopropane was used as a clinical inhalational anesthetic from the 1930s through the 1980s. The substance's high flammability poses a risk of fire and explosions in operating rooms due to its tendency to accumulate in confined spaces, as its density is higher than that of air. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-Dibromopropane, 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiopeptide
Thiopeptides (thiazolyl peptides) are a class of peptide antibiotics produced by bacteria. They have antibiotic activity against Gram-positive bacteria, but little or no activity against Gram-negative bacteria. Many of the members of this class show activity against methicillin-resistant ''Staphylococcus aureus'' (MRSA) and are therefore subjects of research interest. There are over 100 members of this class known. __TOC__ Chemical structure Thiopeptides are sulfur-rich macrocyclic peptides containing highly-modified amino acids. They are characterized by a nitrogen-containing six-membered ring (such as piperidine, dehydropiperidine, or pyridine) substituted with multiple thiazole rings and dehydroamino acids. A macrocylic ring serves as a scaffold for a tail that also incorporates modified amino acids often with azole Azoles are a class of five-membered heterocyclic compounds containing a nitrogen atom and at least one other non-carbon atom (i.e. nitrogen, sulfur, or oxyge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coproporphyrinogen III Oxidase
Coproporphyrinogen-III oxidase, mitochondrial (abbreviated as CPOX) is an enzyme that in humans is encoded by the ''CPOX'' gene. A genetic defect in the enzyme results in a reduced production of heme in animals. The medical condition associated with this enzyme defect is called hereditary coproporphyria. CPOX, the sixth enzyme of the haem biosynthetic pathway, converts coproporphyrinogen III to protoporphyrinogen IX through two sequential steps of oxidative decarboxylation. The activity of the CPOX enzyme, located in the mitochondrial membrane, is measured in lymphocytes. Function CPOX is an enzyme involved in the sixth step of porphyrin metabolism it catalyses the oxidative decarboxylation of coproporphyrinogen III to proto-porphyrinogen IX in the haem and chlorophyll biosynthetic pathways. The protein is a homodimer containing two internally bound iron atoms per molecule of native protein. The enzyme is active in the presence of molecular oxygen that acts as an elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
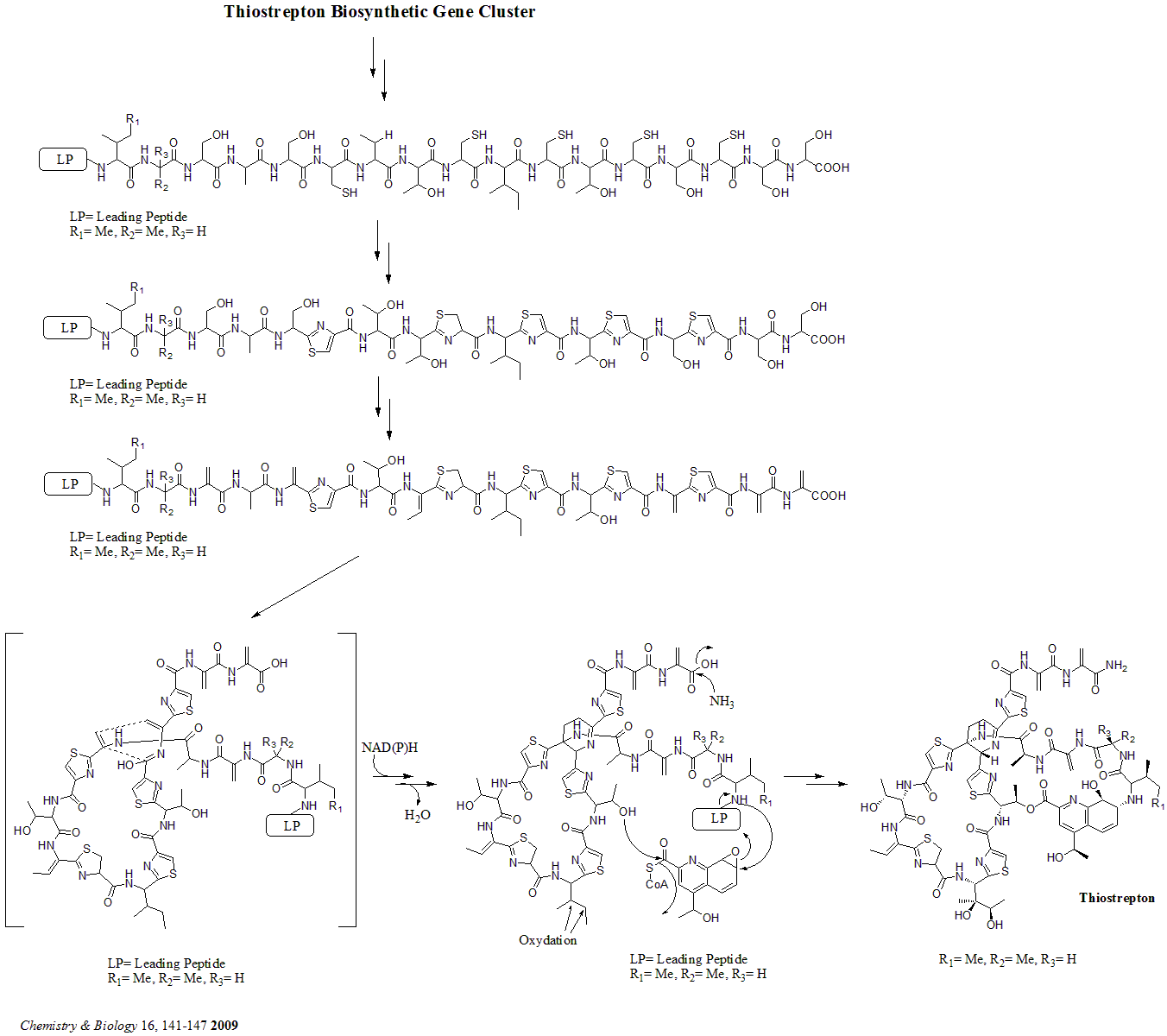
Thiostrepton
Thiostrepton is a natural cyclic oligopeptide antibiotic of the thiopeptide class, derived from several strains of streptomycetes, such as '' Streptomyces azureus'' and '' Streptomyces laurentii''. Thiostrepton is a natural product of the ribosomally synthesized and post-translationally modified peptide (RiPP) class. History Thiostrepton was discovered by Donovick ''et al.'' who described its antibacterial properties in 1955. Dorothy Crowfoot Hodgkin solved the structure of thiostrepton in 1970. Early in 1978, Bycroft and Gowland proposed the biosynthesis of thiostrepton, which was still unclear until 2009. Several studies of thiopeptide biosynthesis have been contemporarily published in 2009 and two of them (Liao ''et al.'' and Kelly ''et al.'') included the similar biosynthesis of thiostrepton: it's ribosomally synthesized from thiostrepton biosynthetic genes (tsr genes) and posttranslational modification is needed. A total synthesis of thiostrepton was completed by K.C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptophan
Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3 (niacin). It is encoded by the codon UGG. Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38). Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid. Tryptophan is named after the digestive enzymes trypsin, which were used in its first isolation from casein proteins. It was assigned the one-letter symbol W based on the double ring being visually suggestive to the bulky letter. Function ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin B12
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. One of eight B vitamins, it serves as a vital cofactor (biochemistry), cofactor in DNA synthesis and both fatty acid metabolism, fatty acid and amino acid metabolism. It plays an essential role in the nervous system by supporting myelinogenesis, myelin synthesis and is critical for the maturation of red blood cells in the bone marrow. While animals require B12, plants do not, relying instead on alternative enzymatic pathways. Vitamin B12 is the most chemically complex of all vitamins, and is synthesized exclusively by certain archaea and bacteria. Natural food sources include meat, shellfish, liver, fish, poultry, Egg as food, eggs, and dairy products. It is also added to many breakfast cereals through food fortification and is available in dietary supplement and pharmaceutical forms. Supplements are commonly taken orally but may be administered via intramuscular injection to treat defic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |