|
Quaternary Compound
In chemistry, a quaternary compound is a compound consisting of exactly four chemical elements. In another use of the term in organic chemistry, a quaternary compound is or has a cation consisting of a central positively charged atom with four substituents, especially organic (alkyl and aryl) groups, discounting hydrogen atoms. The best-known quaternary compounds are quaternary ammonium salts, having a nitrogen atom at the center. For example, in the following reaction, the nitrogen atom is said to be quaternized as it has gone from 3 to 4 substituents: :R3N + RCl -> R4N+Cl- Other examples include substituted phosphonium salts (), substituted arsonium salts () like arsenobetaine, as well as some arsenic-containing superconductors. Substituted stibonium () and bismuthonium salts () have also been described. See also *Binary compound *Ternary compound *Onium ion In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Quaternary Ammonium Cation
In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure , where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion () and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces than bleach-based disinfectants, and are generally fabric-safe. Synthes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Arsonium Salt
The arsonium cation is a positively charged polyatomic ion with the chemical formula . An arsonium salt is a salt containing either the arsonium () cation, such as arsonium bromide () and arsonium iodide (), which can be synthesized by reacting arsine with hydrogen bromide or hydrogen iodide Hydrogen iodide (HI) is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas und ....Muñoz-Hernández, M. Á. (2006). Arsenic: Inorganic Chemistry. ''Encyclopedia of Inorganic Chemistry''. pp 4. DOI: 10.1002/0470862106.ia013 Or more commonly, as organic derivative such as the quaternary arsonium salts (CAS: , hydrate form) and the zwitterionic compound arsenobetaine. References Arsenic(−III) compounds Cations {{chem-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Onium Ion
In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride of a pnictogen (group 15 of the periodic table), chalcogen (group 16), or halogen (group 17). The oldest-known onium ion, and the namesake for the class, is ammonium, , the protonated derivative of ammonia, . The name onium is also used for cations that would result from the substitution of hydrogen atoms in those ions by other groups, such as organic groups, or halogens; such as tetraphenylphosphonium, . The substituent groups may be divalent or trivalent, yielding ions such as iminium and nitrilium. A simple onium ion has a charge of +1. A larger ion that has two onium ion subgroups is called a double onium ion, and has a charge of +2. A triple onium ion has a charge of +3, and so on. Compounds of an onium cation and some other anion are known as onium compounds or onium salts. Onium ions and onium compounds are inversely analogous to ions and ate complexe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
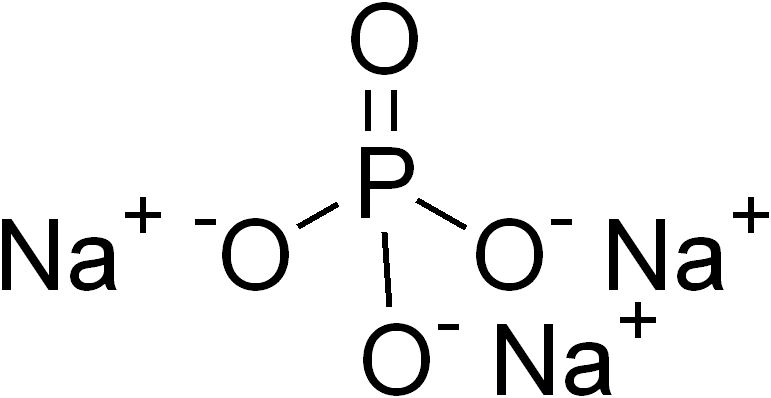
Ternary Compound
In inorganic chemistry and materials chemistry, a ternary compound or ternary phase is a chemical compound containing three different elements. While some ternary compounds are molecular, ''e.g.'' chloroform (), more typically ternary phases refer to extended solids. The perovskites are a famous example. Binary phases, with only two elements, have lower degrees of complexity than ternary phases. With four elements, quaternary phases are more complex. The number of isomers of a ternary compound provide a distinction between inorganic and organic chemistry: "In inorganic chemistry one or, at most, only a few compounds composed of any two or three elements were known, whereas in organic chemistry the situation was very different." Ternary crystalline compounds An example is sodium phosphate, . The sodium ion has a charge of 1+ and the phosphate ion has a charge of 3–. Therefore, three sodium ions are needed to balance the charge of one phosphate ion. Another example of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Binary Compound
In materials chemistry, a binary phase or binary compound is a chemical compound containing two different elements. Some binary phase compounds are molecular, e.g. carbon tetrachloride (CCl4). More typically binary phase refers to extended solids. Famous examples zinc sulfide Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various i ..., which contains zinc and sulfur, and tungsten carbide, which contains tungsten and carbon. Phases with higher degrees of complexity feature more elements, e.g. three elements in ternary phases, four elements in quaternary phases. These phases exhibit a higher degree of complexity due to the interaction of these elements at different conditions. References Chemical compounds {{chem-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Arsenic
Arsenic is a chemical element; it has Symbol (chemistry), symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is notoriously toxic. It occurs naturally in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. It has various Allotropes of arsenic, allotropes, but only the grey form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition). Arsenic is also a common n-type dopant in semiconductor electronic devices, and a component of the III–V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides, treated wood products, herbicides, and insecticides. These applications are declining with the increasing recognition of the persistent tox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Arsenobetaine
Arsenobetaine is an organoarsenic compound that is the main source of arsenic found in fish. It is the arsenic analog of trimethylglycine, commonly known as betaine. The biochemistry and its biosynthesis are similar to those of choline and betaine. Arsenobetaine is a common substance in marine biological systems and unlike many other organoarsenic compounds, such as trimethylarsine, it is relatively non-toxic. The compound may play a similar role as urea does for nitrogen, as a non-toxic waste compound made in the bodies of animals to dispose of the relevant element. It has been known since 1920 that marine fish contain organoarsenic compounds, but it was not until 1977 that the chemical structure of the most predominant compound arsenobetaine was determined. Safety Whereas arsenous acid (As(OH)3) has an LD50 In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phosphonium Salt
In chemistry, the term phosphonium (more obscurely: phosphinium) describes polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, organyl or halogen group). These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions. Types of phosphonium cations Protonated phosphines The parent phosphonium is as found in the iodide salt, phosphonium iodide. Salts of the parent are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakis(hydroxymethyl)phosphonium chloride: :PH3 + HCl + 4 CH2O → Many organophosphonium salts are produced by protonation of primary, secondary, and tertiary phosphines: :PR3 + H+ → The basicity of phosphines follows the usual trends, with R = alkyl being more basic than R = aryl. Tetraorganophosphonium cations The most common phosphonium compounds have four organic substituents attached to phosphorus. The quaternary phosp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |