|
Polydispersity Index
In chemistry, the dispersity is a measure of the heterogeneity of sizes of molecules or particles in a mixture. A collection of objects is called uniform if the objects have the same size, shape, or mass. A sample of objects that have an inconsistent size, shape and mass distribution is called non-uniform. The objects can be in any form of chemical dispersion, such as particles in a colloid, droplets in a cloud, crystals in a rock, or polymer macromolecules in a solution or a solid polymer mass. Polymers can be described by molecular mass distribution; a population of particles can be described by size, surface area, and/or mass distribution; and thin films can be described by film thickness distribution. IUPAC has deprecated the use of the term ''polydispersity index'', having replaced it with the term ''dispersity'', represented by the symbol Đ (pronounced D-strokeStepto, R. F. T.; Gilbert, R. G.; Hess, M.; Jenkins, A. D.; Jones, R. G.; Kratochvíl P. (2009).Dispersity in Po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fulvic Acid
Humic substances (HS) are organic compounds that are important components of humus, the major organic fraction of soil, peat, and coal (and also a constituent of many upland streams, dystrophic lakes, and ocean water). For a long era in the 19th and 20th centuries, humic substances were often viewed through a lens of acid–base theory that described humic acids (HA), as organic acids, and their conjugate bases, humates, as important components of organic matter. Through this viewpoint humic acids were defined as organic substances extracted from soil that coagulate (form small solid pieces) when a strong-base extract is acidified, whereas fulvic acids (FA) are organic acids that remain soluble (stay dissolved) when a strong-base extract is acidified. The remaining alkali-insoluble part of humus would be termed humin. Humic matter in isolation is the result of a chemical extraction from the soil organic matter or the dissolved organic matter and represent the humic molecules dist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Damköhler Numbers
The Damköhler numbers (Da) are dimensionless numbers used in chemical engineering to relate the chemical reaction timescale (reaction rate) to the transport phenomena rate occurring in a system. It is named after German chemist Gerhard Damköhler. The Karlovitz number (Ka) is related to the Damköhler number by Da = 1/Ka. In its most commonly used form, the Damköhler number relates the reaction timescale to the convection time scale, volumetric flow rate, through the reactor for continuous (plug flow or stirred tank) or semibatch chemical processes: : \mathrm = \frac In reacting systems that include interphase mass transport, the second Damköhler number (DaII) is defined as the ratio of the chemical reaction rate to the mass transfer rate : \mathrm_ = \frac It is also defined as the ratio of the characteristic fluidic and chemical time scales: : \mathrm = \frac Since the reaction timescale is determined by the reaction rate, the exact formula for the Damköhler number var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geometric Distribution
In probability theory and statistics, the geometric distribution is either one of two discrete probability distributions: * The probability distribution of the number ''X'' of Bernoulli trials needed to get one success, supported on the set \; * The probability distribution of the number ''Y'' = ''X'' − 1 of failures before the first success, supported on the set \. Which of these is called the geometric distribution is a matter of convention and convenience. These two different geometric distributions should not be confused with each other. Often, the name ''shifted'' geometric distribution is adopted for the former one (distribution of the number ''X''); however, to avoid ambiguity, it is considered wise to indicate which is intended, by mentioning the support explicitly. The geometric distribution gives the probability that the first occurrence of success requires ''k'' independent trials, each with success probability ''p''. If the probability of succe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
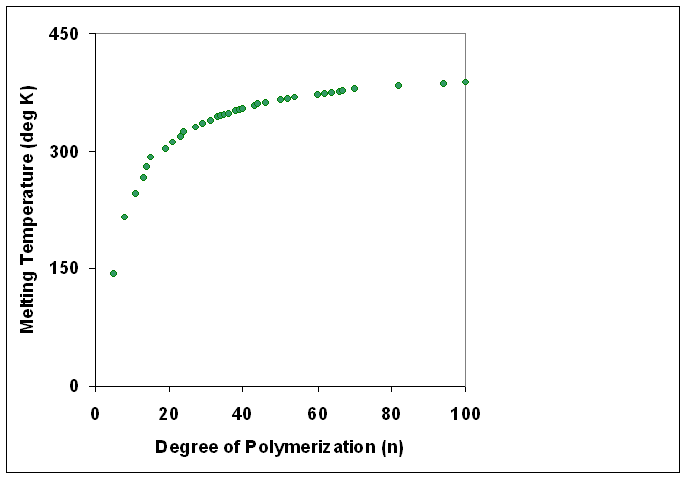
Degree Of Polymerization
The degree of polymerization, or DP, is the number of monomeric units in a macromolecule or polymer or oligomer molecule. For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymerization is given by DP_n\equiv X_n=\frac, where Mn is the number-average molecular weight and M0 is the molecular weight of the monomer unit. For most industrial purposes, degrees of polymerization in the thousands or tens of thousands are desired. This number does not reflect the variation in molecule size of the polymer that typically occurs, it only represents the mean number of monomeric units. Some authors, however, define DP as the number of repeat units, where for copolymers the repeat unit may not be identical to the monomeric unit.Fried J.R. "Polymer Science and Technology" (Pearson Prentice-Hall, 2nd edn 2003), p.27 For example, in nylon-6,6, the repeat unit contains the two monomeric units —NH(CH2)6NH— and —OC(CH2)4CO—, so that a ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Continuous Stirred-tank Reactor
The continuous stirred-tank reactor (CSTR), also known as vat- or backmix reactor, mixed flow reactor (MFR), or a continuous-''flow'' stirred-tank reactor (C''F''STR), is a common model for a chemical reactor in chemical engineering and environmental engineering. A CSTR often refers to a model used to estimate the key unit operation variables when using a continuous agitated-tank reactor to reach a specified output. The mathematical model works for all fluids: liquids, gases, and slurries. The behavior of a CSTR is often approximated or modeled by that of an ideal CSTR, which assumes perfect mixing. In a perfectly mixed reactor, reagent is instantaneously and uniformly mixed throughout the reactor upon entry. Consequently, the output composition is identical to composition of the material inside the reactor, which is a function of residence time and reaction rate. The CSTR is the ideal limit of complete mixing in reactor design, which is the complete opposite of a plug flow reacto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plug Flow Reactor Model
The plug flow reactor model (PFR, sometimes called continuous tubular reactor, CTR, or piston flow reactors) is a model used to describe chemical reactions in continuous, flowing systems of cylindrical geometry. The PFR model is used to predict the behavior of chemical reactors of such design, so that key reactor variables, such as the dimensions of the reactor, can be estimated. Fluid going through a PFR may be modeled as flowing through the reactor as a series of infinitely thin coherent "plugs", each with a uniform composition, traveling in the axial direction of the reactor, with each plug having a different composition from the ones before and after it. The key assumption is that as a plug flows through a PFR, the fluid is perfectly mixed in the radial direction but not in the axial direction (forwards or backwards). Each plug of differential volume is considered as a separate entity, effectively an infinitesimally small continuous stirred tank reactor, limiting to zero vo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Living Polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar (i.e. they have a very low polydispersity index). Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups. Living polymerization is desirable because it offers precision and control in macromolecular synthesis. This is important since many of the novel/useful properties of polymers result ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carothers' Equation
In step-growth polymerization, the Carothers equation (or Carothers' equation) gives the degree of polymerization, , for a given fractional monomer conversion, . There are several versions of this equation, proposed by Wallace Carothers, who invented nylon in 1935. Linear polymers: two monomers in equimolar quantities The simplest case refers to the formation of a strictly linear polymer by the reaction (usually by condensation) of two monomers in equimolar quantities. An example is the synthesis of nylon-6,6 whose formula is from one mole of hexamethylenediamine, , and one mole of adipic acid, . For this case :\bar_n=\frac In this equation * is the number-average value of the degree of polymerization, equal to the average number of monomer units in a polymer molecule. For the example of nylon-6,6 \bar_n = 2n ( diamine units and diacid units). *p=\tfrac is the extent of reaction (or conversion to polymer), defined by ** is the number of molecules present initially as monom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them. In chemical compounds, polymerization can occur via a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects. In more straightforward polymerizations, alkenes form polymers through relatively simple radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize. Alkanes can also be polymerized, but only with the help of strong acids. As alkenes can polymerize in somewhat straightforward radical reactions, they form useful compounds such as polyethylene and polyvinyl chloride (PV ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactant
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, '' catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reage ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Number Average Molecular Weight
The molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer species (Ni) and the molar mass (Mi) of that species. In linear polymers, the individual polymer chains rarely have exactly the same degree of polymerization and molar mass, and there is always a distribution around an average value. The molar mass distribution of a polymer may be modified by polymer fractionation. Definitions of molar mass average Different average values can be defined, depending on the statistical method applied. In practice, four averages are used, representing the weighted mean taken with the mole fraction, the weight fraction, and two other functions which can be related to measured quantities: *''Number average molar mass'' (Mn), also loosely referred to as ''number average molecular weight'' (NAMW). *''Mass average molar mass'' (Mw), where ''w'' stands for weight; also commonly referred to as ''weight average'' or ''weigh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |