|
Photoluminescence
Photoluminescence (abbreviated as PL) is light emission from any form of matter after the absorption of photons (electromagnetic radiation). It is one of many forms of luminescence (light emission) and is initiated by photoexcitation (i.e. photons that excite electrons to a higher energy level in an atom), hence the prefix ''photo-''. Following excitation, various relaxation processes typically occur in which other photons are re-radiated. Time periods between absorption and emission may vary: ranging from short femtosecond-regime for emission involving free-carrier plasma in inorganic semiconductorsHayes, G.R.; Deveaud, B. (2002). "Is Luminescence from Quantum Wells Due to Excitons?". ''Physica Status Solidi A'' 190 (3): 637–640doi:10.1002/1521-396X(200204)190:33.0.CO;2-7/ref> up to milliseconds for phosphoresence processes in molecular systems; and under special circumstances delay of emission may even span to minutes or hours. Observation of photoluminescence at a certain e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoluminescence Animation
Photoluminescence (abbreviated as PL) is light emission from any form of matter after the absorption of photons (electromagnetic radiation). It is one of many forms of luminescence (light emission) and is initiated by photoexcitation (i.e. photons that excite electrons to a higher energy level in an atom), hence the prefix ''photo-''. Following excitation, various relaxation processes typically occur in which other photons are re-radiated. Time periods between absorption and emission may vary: ranging from short femtosecond-regime for emission involving free-carrier plasma in inorganic semiconductorsHayes, G.R.; Deveaud, B. (2002). "Is Luminescence from Quantum Wells Due to Excitons?". ''Physica Status Solidi A'' 190 (3): 637–640doi:10.1002/1521-396X(200204)190:33.0.CO;2-7/ref> up to milliseconds for phosphoresence processes in molecular systems; and under special circumstances delay of emission may even span to minutes or hours. Observation of photoluminescence at a certain en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Luminescence
Luminescence is spontaneous emission of light by a substance not resulting from heat; or "cold light". It is thus a form of cold-body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a substance as a result of heating. Historically, radioactivity was thought of as a form of "radio-luminescence", although it is today considered to be separate since it involves more than electromagnetic radiation. The dials, hands, scales, and signs of aviation and navigational instruments and markings are often coated with luminescent materials in a process known as "luminising". Types The following are types of luminescence: * Chemiluminescence, the emission of light as a result of a chemical reaction ** Bioluminescence, a result of biochemical reactions in a living organism **Electrochemiluminescence, a result of an electrochemical reaction **Lyolum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
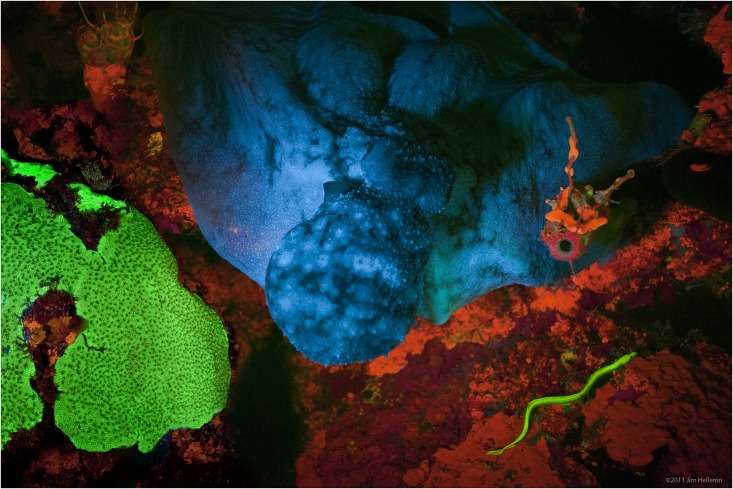
Fluorescence Rainbow
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vacu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoresence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed. In a general sense, there is no distinct boundary between the emission times of fluorescence and phosphorescence (i.e.: if a substance glows under a black light it is generally considered fluorescent, and if it glows in the dark it is often simply called phosphorescent). In a modern, scientific sense, the phenomena can usually be classified by the three different mechanisms that produce the light, and the typical timescales during which those mechanisms emit light. Whereas fluorescent materials stop emitti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed. In a general sense, there is no distinct boundary between the emission times of fluorescence and phosphorescence (i.e.: if a substance glows under a black light it is generally considered fluorescent, and if it glows in the dark it is often simply called phosphorescent). In a modern, scientific sense, the phenomena can usually be classified by the three different mechanisms that produce the light, and the typical timescales during which those mechanisms emit light. Whereas fluorescent materials stop emit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bandgap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in electron volts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electron bound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there is no generated current due to no net charge carrier mobility. However, if some electrons transfer fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Compendium Of Chemical Terminology
The International Union of Pure and Applied Chemistry publishes many books which contain its complete list of definitions. The definitions are divided into seven "colour books": Gold, Green, Blue, Purple, Orange, White, and Red. There is also an eighth book—the "Silver Book". The eight ''colour books'' ''Blue Book'' ''Nomenclature of Organic Chemistry'', commonly referred to by chemists as the ''Blue Book'', is a collection of recommendations on organic chemical nomenclature published at irregular intervals by the International Union of Pure and Applied Chemistry (IUPAC). A full edition was published in 1979, an abridged and updated version of which was published in 1993 as ''A Guide to IUPAC Nomenclature of Organic Compounds''. Both of these are now out-of-print in their paper versions, but are available free of charge in electronic versions. After the release of a draft version for public comment in 2004 and the publication of several revised sections in the journal ''Pure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work ( pharmacology), and how to collec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GaAs
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a zinc blende crystal structure. Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monolithic microwave integrated circuits, infrared light-emitting diodes, laser diodes, solar cells and optical windows. GaAs is often used as a substrate material for the epitaxial growth of other III-V semiconductors, including indium gallium arsenide, aluminum gallium arsenide and others. Preparation and chemistry In the compound, gallium has a +3 oxidation state. Gallium arsenide single crystals can be prepared by three industrial processes: * The vertical gradient freeze (VGF) process. * Crystal growth using a horizontal zone furnace in the Bridgman-Stockbarger technique, in which gallium and arsenic vapors react, and free molecules deposit on a seed crystal at the cooler end of the furnace. * Liquid encapsulated Czochralski (LEC) growth is used for producing high-pu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Arsenide
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a zinc blende crystal structure. Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monolithic microwave integrated circuits, infrared light-emitting diodes, laser diodes, solar cells and optical windows. GaAs is often used as a substrate material for the epitaxial growth of other III-V semiconductors, including indium gallium arsenide, aluminum gallium arsenide and others. Preparation and chemistry In the compound, gallium has a +3 oxidation state. Gallium arsenide single crystals can be prepared by three industrial processes: * The vertical gradient freeze (VGF) process. * Crystal growth using a horizontal zone furnace in the Bridgman-Stockbarger technique, in which gallium and arsenic vapors react, and free molecules deposit on a seed crystal at the cooler end of the furnace. * Liquid encapsulated Czochralski (LEC) growth is used for producing hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Minority Carrier Lifetime
A definition in semiconductor physics, carrier lifetime is defined as the average time it takes for a minority carrier to Carrier generation and recombination, recombine. The process through which this is done is typically known as minority carrier recombination. The energy released due to Carrier generation and recombination, recombination can be either thermal, thereby heating up the semiconductor (''thermal recombination'' or non-radiative recombination, one of the sources of waste heat in semiconductors), or released as photons (''optical recombination'', used in light-emitting diode, LEDs and semiconductor lasers). The carrier lifetime can vary significantly depending on the materials and construction of the semiconductor. Carrier lifetime plays an important role in Bipolar junction transistor, bipolar transistors and solar cells. In indirect band gap semiconductors, the carrier lifetime strongly depends on the concentration of recombination centers. Gold atoms act as hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |