|
Pydiflumetofen
Pydiflumetofen belongs to the large family of SDHI pesticides, it is used as broad spectrum fungicide in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2016 using their brand name Miravis. The compound is an methoxyamide which combines a 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid, pyrazole acid with a substituted amphetamine to give an inhibitor of succinate dehydrogenase, an enzyme that is important to cellular respiration in almost all living organisms. History Inhibition of succinate dehydrogenase, the complex II in the mitochondrial respiration chain, has been known as a fungicidal mechanism of action since the first examples were marketed in the 1960s. The first compound in this class was carboxin, which had a narrow spectrum of useful biological activity, mainly on basidiomycetes and was used as a seed treatment. By 2016, at least 17 further examples of this mechanism of action were developed by crop protection companie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid
3-(Difluoromethyl)-1-methyl-1''H''-pyrazole-4-carboxylic acid is a chemical compound which is used commercially as an intermediate to seven fungicides which act by inhibition of succinate dehydrogenase (SDHI). It consists of a pyrazole ring with difluoromethyl, methyl and carboxylic acid groups attached in specific positions. Background Inhibition of succinate dehydrogenase, the complex II in the mitochondrial respiration chain, has been known as a fungicidal mechanism of action since the first examples were marketed in the 1960s. By 2016, at least 18 examples were developed by crop protection companies, with the market leader being boscalid, owing to its broad spectrum of fungal species controlled. However, it lacked full control of important cereal diseases, especially septoria leaf blotch ''Zymoseptoria tritici''. A group of compounds which did control septoria were 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic amides, as shown below, ordered by year of their first reg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Syngenta
Syngenta Global AG is a global agricultural technology company headquartered in Basel, Switzerland. It primarily covers crop protection and seeds for farmers. Syngenta is part of the Syngenta Group, entirely owned by Sinochem, a Chinese state-owned enterprise. Syngenta was founded in 2000 by the merger of the agrichemical businesses of Novartis and AstraZeneca, and acquired by China National Chemical Corporation (ChemChina) in 2017. In 2020, the Syngenta Group was formed, bringing together Syngenta Crop Protection and Syngenta Seeds, Adama, and the agricultural business of Sinochem, now called Syngenta Group China, under a single entity. Syngenta's primary products include pesticides, selective herbicides, non-selective herbicides, fungicides, insecticides, as well as corn, soya, and biofuel. Syngenta brands include Actara ( Thiamethoxam), Agrisure (corn with Viptera trait), Alto ( Cyproconazole), Amistar ( azoxystrobin), Avicta, Axial, Bicep II, Bravo, Callisto, Celest, C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ketoxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides () with general structure . Oximes are usually generated by the reaction of hydroxylamine with aldehydes () or ketones (). The term ''oxime'' dates back to the 19th century, a combination of the words ''oxygen'' and ''imine''. Structure and properties If the two side-chains on the central carbon are different from each other—either an aldoxime, or a ketoxime with two different "R" groups—the oxime can often have two different geometric stereoisomeric forms according to the ''E''/''Z'' configuration. An older terminology of ''syn'' and ''anti'' was used to identify especially aldoximes according to whether the R group was closer or further from the hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Reduction (chemistry)
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron transfer, Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one Substrate (chemistry), substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nitroethane
Nitroethane is an organic compound having the chemical formula C2H5NO2. Similar in many regards to nitromethane, nitroethane is an oily liquid at standard temperature and pressure. Pure nitroethane is colorless and has a fruity odor. Preparation Nitroethane is produced industrially by treating propane with nitric acid at 350–450 °C. This exothermic reaction produces four industrially significant nitroalkanes: nitromethane, nitroethane, 1-nitropropane, and 2-Nitropropane, 2-nitropropane. The reaction involves free radicals, such as CH3CH2CH2O., which arise via homolysis of the corresponding nitrite ester. These alkoxy radicals are susceptible to C—C fragmentation reactions, which explains the formation of a mixture of products.Sheldon B. Markofsky “Nitro Compounds, Aliphatic” in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2002. . Alternatively, nitroethane can be produced by the Victor Meyer reaction of haloethanes such as chloroeth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
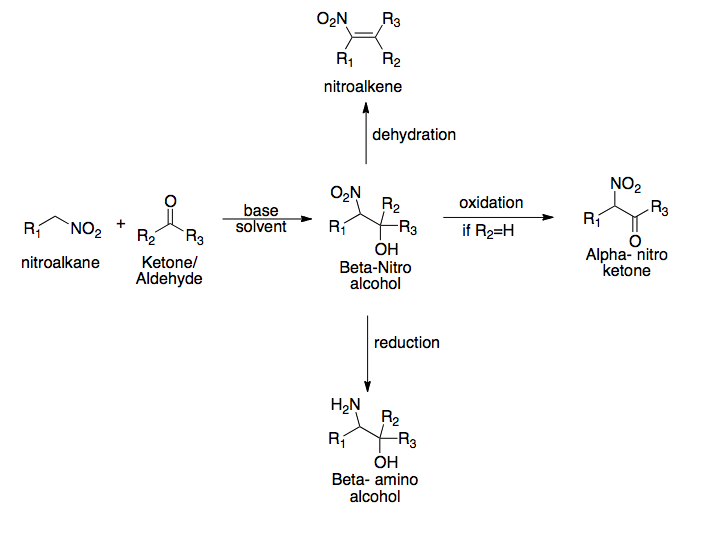
Henry Reaction
The Henry reaction is a classic carbon–carbon bond formation reaction in organic chemistry. Discovered in 1895 by the Belgian chemist Louis Henry (1834–1913), it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-nitro alcohols. This type of reaction is also referred to as a nitroaldol reaction (nitroalkane, aldehyde, and alcohol). It is nearly analogous to the aldol reaction that had been discovered 23 years prior that couples two carbonyl compounds to form β-hydroxy carbonyl compounds known as "aldols" (aldehyde and alcohol). The Henry reaction is a useful technique in the area of organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates. These conversions include subsequent dehydration to yield nitroalkenes, oxidation of the secondary alcohol to yield α-nitro ketones, or reduction of the nitro group to yield β-amino alcohols. Man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Sedaxane
Sedaxane is a broad spectrum fungicide used as a seed treatment in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2011 using their brand name Vibrance. The compound is an amide which combines a pyrazole acid with an aryl amine to give an inhibitor of succinate dehydrogenase. The compound is widely registered for use, including in Australia, the EU, UK and US. History Inhibition of succinate dehydrogenase, the complex II in the mitochondrial respiration chain, has been known as a fungicidal mechanism of action since the first examples were marketed in the 1960s. The first compound in this class was carboxin, which had a narrow spectrum of useful biological activity, mainly on basidiomycetes and was used as a seed treatment. By 2016, at least 17 further examples of this mechanism of action were developed by crop protection companies, with the market leader being boscalid, owing to its broader spectrum of fungal species controlled. However, it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |