|
Protein Fragment Library
Protein backbone fragment libraries have been used successfully in a variety of structural biology applications, including homology modeling,Kolodny, R., Guibas, L., Levitt, M., and Koehl, P. (2005, March). Inverse Kinematics in Biology: The Protein Loop Closure Problem. The International Journal of Robotics Research 24(2-3), 151-163. de novo structure prediction, Simons, K., Kooperberg, C., Huang, E., and Baker, D. (1997). Assembly of Protein Tertiary Structures from Fragments with Similar Local Sequences using Simulated Annealing and Bayesian Scoring Functions. J Mol Biol 268, 209-225.Bujnicki, J. (2006) Protein Structure Prediction by Recombination of Fragments. ChemBioChem. 7, 19-27.Li, S. et al. (2008) Fragment-HMM: A New Approach to Protein Structure Prediction. Protein Science. 17, 1925-1934. and structure determination.DiMaio, F., Shavlik, J., Phillips, G. A probabilistic approach to protein backbone tracing in electron density maps (2006). Bioinformatics 22(14), 81-89. By ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Biology
Structural biology deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every level of organization. Early structural biologists throughout the 19th and early 20th centuries were primarily only able to study structures to the limit of the naked eye's visual acuity and through magnifying glasses and light microscopes. In the 20th century, a variety of experimental techniques were developed to examine the 3D structures of biological molecules. The most prominent techniques are X-ray crystallography, nuclear magnetic resonance, and Electron microscope, electron microscopy. Through the discovery of X-ray, X-rays and its applications to protein crystals, structural biology was revolutionized, as now scientists could obtain the three-dimensional structures of biological molecules in atomic detail. Likewise, Nuclear magnetic resonance spectroscopy, NMR spectroscopy allowed information about protein structure and dynamic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
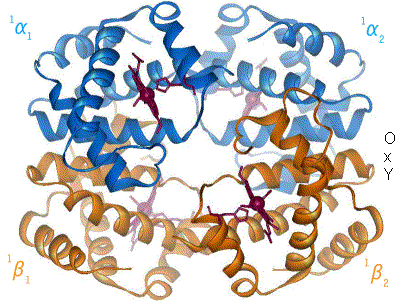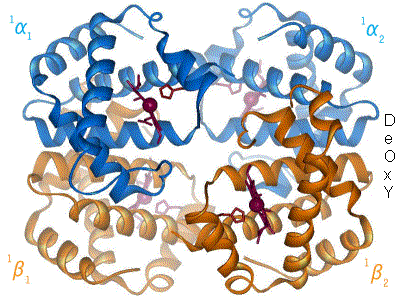
Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules such as proteins and nucleic acids, which is overseen by the Worldwide Protein Data Bank (wwPDB). This structural data is obtained and deposited by biologists and biochemists worldwide through the use of experimental methodologies such as X-ray crystallography, Nuclear magnetic resonance spectroscopy of proteins, NMR spectroscopy, and, increasingly, cryo-electron microscopy. All submitted data are reviewed by expert Biocuration, biocurators and, once approved, are made freely available on the Internet under the CC0 Public Domain Dedication. Global access to the data is provided by the websites of the wwPDB member organizations (PDBe, PDBj, RCSB PDB, and BMRB). The PDB is a key in areas of structural biology, such as structural genomics. Most major scientific journals and some funding agencies now require scientists to submit their structure data to the PDB. Many other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Alignment
Structural alignment attempts to establish homology between two or more polymer structures based on their shape and three-dimensional conformation. This process is usually applied to protein tertiary structures but can also be used for large RNA molecules. In contrast to simple structural superposition, where at least some equivalent residues of the two structures are known, structural alignment requires no ''a priori'' knowledge of equivalent positions. Structural alignment is a valuable tool for the comparison of proteins with low sequence similarity, where evolutionary relationships between proteins cannot be easily detected by standard sequence alignment techniques. Structural alignment can therefore be used to imply evolutionary relationships between proteins that share very little common sequence. However, caution should be used in using the results as evidence for shared evolutionary ancestry because of the possible confounding effects of convergent evolution by which mul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Structure Prediction Software
This list of protein structure prediction software summarizes notable used software tools in protein structure prediction, including homology modeling, protein threading, ''ab initio'' methods, Protein structure prediction#Secondary structure, secondary structure prediction, and transmembrane helix and signal peptide prediction. Software list Below is a list which separates programs according to the method used for structure prediction. Homology modeling Threading and fold recognition ''Ab initio'' structure prediction Secondary structure prediction Detailed list of programs can be found at List of protein secondary structure prediction programs See also *List of protein secondary structure prediction programs *Comparison of nucleic acid simulation software *List of software for molecular mechanics modeling *Molecular design software *Protein design *AlphaFold External linksbio.tools finding more tools References {{DEFAULTSORT:Protein structure predictio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Structure Prediction
Protein structure prediction is the inference of the three-dimensional structure of a protein from its amino acid sequence—that is, the prediction of its Protein secondary structure, secondary and Protein tertiary structure, tertiary structure from Protein primary structure, primary structure. Structure prediction is different from the inverse problem of protein design. Protein structure prediction is one of the most important goals pursued by computational biology and addresses Levinthal's paradox. Accurate structure prediction has important applications in medicine (for example, in drug design) and biotechnology (for example, in novel enzyme design). Starting in 1994, the performance of current methods is assessed biannually in the ''Critical Assessment of Structure Prediction'' (CASP) experiment. A continuous evaluation of protein structure prediction web servers is performed by the community project ''Continuous Automated Model EvaluatiOn'' (CAMEO3D). Protein structure a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Design
Protein design is the rational design of new protein molecules to design novel activity, behavior, or purpose, and to advance basic understanding of protein function. Proteins can be designed from scratch (''de novo'' design) or by making calculated variants of a known protein structure and its sequence (termed ''protein redesign''). Rational protein design approaches make protein-sequence predictions that will fold to specific structures. These predicted sequences can then be validated experimentally through methods such as peptide synthesis, site-directed mutagenesis, or artificial gene synthesis. Rational protein design dates back to the mid-1970s. Recently, however, there were numerous examples of successful rational design of water-soluble and even transmembrane peptides and proteins, in part due to a better understanding of different factors contributing to protein structure stability and development of better computational methods. Overview and history The goal in ratio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homology Modeling
Homology modeling, also known as comparative modeling of protein, refers to constructing an atomic-resolution model of the "''target''" protein from its amino acid sequence and an experimental three-dimensional structure of a related homologous protein (the "''template''"). Homology modeling relies on the identification of one or more known protein structures likely to resemble the structure of the query sequence, and on the production of a sequence alignment that maps residues in the query sequence to residues in the template sequence. It has been seen that protein structures are more conserved than protein sequences amongst homologues, but sequences falling below a 20% sequence identity can have very different structure. Evolutionarily related proteins have similar sequences and naturally occurring homologous proteins have similar protein structure. It has been shown that three-dimensional protein structure is evolutionarily more conserved than would be expected on the basis of s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NP-hard
In computational complexity theory, a computational problem ''H'' is called NP-hard if, for every problem ''L'' which can be solved in non-deterministic polynomial-time, there is a polynomial-time reduction from ''L'' to ''H''. That is, assuming a solution for ''H'' takes 1 unit time, ''H''s solution can be used to solve ''L'' in polynomial time. As a consequence, finding a polynomial time algorithm to solve a single NP-hard problem would give polynomial time algorithms for all the problems in the complexity class NP. As it is suspected, but unproven, that P≠NP, it is unlikely that any polynomial-time algorithms for NP-hard problems exist. A simple example of an NP-hard problem is the subset sum problem. Informally, if ''H'' is NP-hard, then it is at least as difficult to solve as the problems in NP. However, the opposite direction is not true: some problems are undecidable, and therefore even more difficult to solve than all problems in NP, but they are probably not NP- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Threading
In molecular biology, protein threading, also known as fold recognition, is a method of protein modeling which is used to model those proteins which have the same fold as proteins of known structures, but do not have homologous proteins with known structure. It differs from the homology modeling method of structure prediction as it (protein threading) is used for proteins which do not have their homologous protein structures deposited in the Protein Data Bank (PDB), whereas homology modeling is used for those proteins which do. Threading works by using statistical knowledge of the relationship between the structures deposited in the PDB and the sequence of the protein which one wishes to model. The prediction is made by "threading" (i.e. placing, aligning) each amino acid in the target sequence to a position in the template structure, and evaluating how well the target fits the template. After the best-fit template is selected, the structural model of the sequence is built base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Sheet
The beta sheet (β-sheet, also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, Alzheimer's disease and other proteinopathies. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids in order to build accurate models, especially since he did not then know that the peptide bond was planar. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Helix
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix). The alpha helix is the most common structural arrangement in the Protein secondary structure, secondary structure of proteins. It is also the most extreme type of local structure, and it is the local structure that is most easily predicted from a sequence of amino acids. The alpha helix has a right-handed helix conformation in which every backbone amino, N−H group hydrogen bonds to the backbone carbonyl, C=O group of the amino acid that is four residue (biochemistry), residues earlier in the protein sequence. Other names The alpha helix is also commonly called a: * Pauling–Corey–Branson α-helix (from the names of three scientists who described its structure) * 3.613-helix because there are 3.6 amino acids in one ring, with 13 atoms being involved in the ring formed by the hydrogen bond (starting with amidic hydrogen and ending with carbonyl oxygen) Discovery ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |