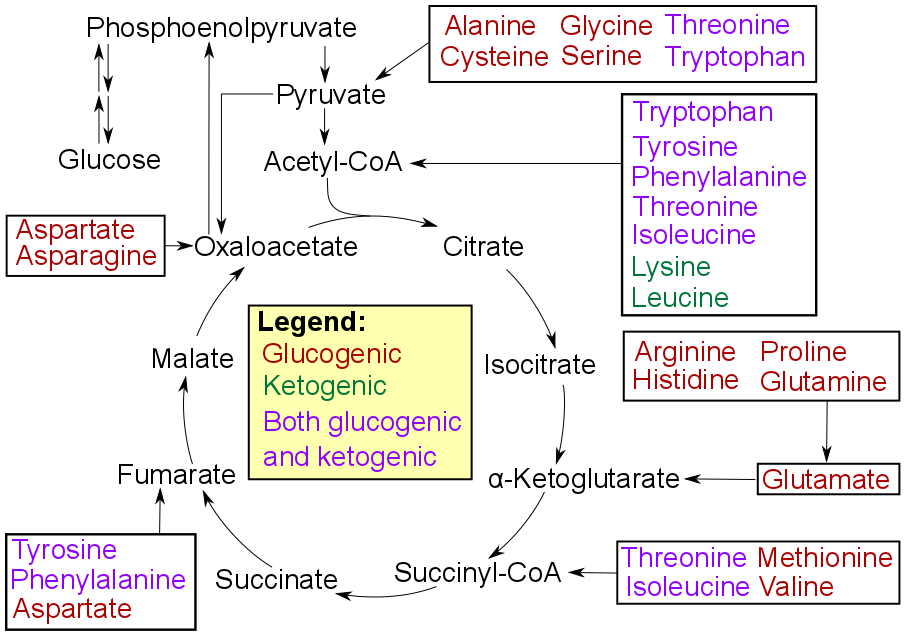
|
Protein Catabolism
In molecular biology, protein catabolism is the breakdown of proteins into smaller peptides and ultimately into amino acids. Protein catabolism is a key function of digestion process. Protein catabolism often begins with pepsin, which converts proteins into polypeptides. These polypeptides are then further degraded. In humans, the pancreatic proteases include trypsin, chymotrypsin, and other enzymes. In the intestine, the small peptides are broken down into amino acids that can be absorbed into the bloodstream. These absorbed amino acids can then undergo amino acid catabolism, where they are utilized as an energy source or as precursors to new proteins. The amino acids produced by catabolism may be directly recycled to form new proteins, converted into different amino acids, or can undergo amino acid catabolism to be converted to other compounds via the Krebs cycle. Interface with other metabolic and salvage pathways Protein catabolism produces amino acids that are used to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Biology
Molecular biology is a branch of biology that seeks to understand the molecule, molecular basis of biological activity in and between Cell (biology), cells, including biomolecule, biomolecular synthesis, modification, mechanisms, and interactions. Though cells and other microscopic structures had been observed in living organisms as early as the 18th century, a detailed understanding of the mechanisms and interactions governing their behavior did not emerge until the 20th century, when technologies used in physics and chemistry had advanced sufficiently to permit their application in the biological sciences. The term 'molecular biology' was first used in 1945 by the English physicist William Astbury, who described it as an approach focused on discerning the underpinnings of biological phenomena—i.e. uncovering the physical and chemical structures and properties of biological molecules, as well as their interactions with other molecules and how these interactions explain observ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteasome
Proteasomes are essential protein complexes responsible for the degradation of proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases. Proteasomes are found inside all eukaryotes and archaea, and in some bacteria. In eukaryotes, proteasomes are located both in the nucleus and in the cytoplasm. The proteasomal degradation pathway is essential for many cellular processes, including the cell cycle, the regulation of gene expression, and responses to oxidative stress. The importance of proteolytic degradation inside cells and the role of ubiquitin in proteolytic pathways was acknowledged in the award of the 2004 Nobel Prize in Chemistry to Aaron Ciechanover, Avram Hershko and Irwin Rose. The core 20S proteasome (blue in the adjacent figure) is a cylindrical, compartmental protein complex of four stacked rings forming a central pore. Each ring is composed of seven individual proteins. The inner two rings a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-end Rule
The ''N''-end rule is a rule that governs the rate of proteolysis, protein degradation through recognition of the N-terminal residue of proteins. The rule states that the N-terminus, ''N''-terminal amino acid of a protein determines its half-life (time after which half of the total amount of a given polypeptide is degraded). The rule applies to both eukaryotic and prokaryotic organisms, but with different strength, rules, and outcome. In eukaryotic cells, these N-terminal residues are recognized and targeted by ubiquitin ligases, mediating ubiquitination thereby marking the protein for degradation. The rule was initially discovered by Alexander Varshavsky and co-workers in 1986. However, only rough estimations of protein half-life can be deduced from this 'rule', as N-terminal amino acid modification can lead to variability and anomalies, whilst amino acid impact can also change from organism to organism. Other degradation signals, known as degrons, can also be found in sequence. Ru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transaminase
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins. Function and mechanism An amino acid contains an amino (NH2) group. A keto acid contains a ketone, keto (=O) group. In transamination, the NH2 group on one molecule is exchanged with the =O group on the other molecule. The amino acid becomes a keto acid, and the keto acid becomes an amino acid. Transaminases require the coenzyme pyridoxal phosphate, which is converted into pyridoxamine in the first half-reaction, when an amino acid is converted into a keto acid. Enzyme-bound pyridoxamine in turn reacts with pyruvate, oxaloacetate, or alpha-ketoglutarate, giving alanine, aspartic acid, or glutamic acid, respectively. Many transamination reactions occur in tissues, catalysed by transaminases specific for a particular amino/keto acid pair. The reactions are readily reversible, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxaloacetic Acid
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle. Properties Oxaloacetic acid undergoes successive deprotonations to give the dianion: :HO2CC(O)CH2CO2H −O2CC(O)CH2CO2H + H+, pKa = 2.22 :−O2CC(O)CH2CO2H −O2CC(O)CH2CO2− + H+, pKa = 3.89 At high pH, the enolizable proton is ionized: :−O2CC(O)CH2CO2− −O2CC(O−)CHCO2− + H+, pKa = 13.03 The enol forms of oxaloacetic acid are particularly stable. Keto-enol tautomerization is catalyzed by the enzyme oxaloacetate tautomerase. ''trans''-Enol-oxaloacetate also appears when tartrate is the substrate for fumarase. Biosynthesis Oxaloacetate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamate
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a Essential amino acid, non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons. Its molecular formula is . Glutamic acid exists in two optically isomeric forms; the optical rotation, dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxylic acid, carboxyl groups −COOH and one amine, amino group � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cell (biology), cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known forms of life, it is often referred to as the "molecular unit of currency" for intracellular energy transfer. When consumed in a Metabolism, metabolic process, ATP converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. It is also a Precursor (chemistry), precursor to DNA and RNA, and is used as a coenzyme. An average adult human processes around 50 kilograms (about 100 mole (unit), moles) daily. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base (adenine), the sugar ribose, and the Polyphosphate, triphosphate. Structure ATP consists of three parts: a sugar, an amine base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidation, oxidized for energy production. Coenzyme A (CoASH or CoA) consists of a cysteamine, β-mercaptoethylamine group linked to pantothenic acid (vitamin B5) through an amide linkage and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol). CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through Beta oxidation, β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyruvic Acid
Pyruvic acid (CH3COCOOH) is the simplest of the keto acids, alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate acid, conjugate base, CH3COCOO−, is an metabolic intermediate, intermediate in several metabolic pathways throughout the cell. Pyruvic acid can be made from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or converted to fatty acids through a reaction with acetyl-CoA. It can also be used to construct the amino acid alanine and can be converted into ethanol or lactic acid via fermentation. Pyruvic acid supplies energy to cell (biology), cells through the citric acid cycle (also known as the Krebs cycle) when oxygen is present (aerobic respiration), and alternatively lactic acid fermentation, ferments to produce lactic acid, lactate when oxygen is lacking. Chemistry In 1834, Théophile-Jules Pelouze distilled tartaric acid and isolated glutaric acid and another unknown organi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and NADH, reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes. The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, can occur in the Great Oxygenation Event, oxygen-free conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal ions, meaning this is a plausible prebiotic pathway for abiogenesis. The most common type of glycolysis is the ''Embden–Meyerhof–Parnas (EMP) pathway'', which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Kar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keto Acid
In organic chemistry, keto acids or ketoacids (also called oxo acids or oxoacids) are organic compounds that contain a carboxylic acid group () and a ketone group ().Franz Dietrich Klingler, Wolfgang Ebertz "Oxocarboxylic Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. In several cases, the keto group is hydrated. The alpha-keto acids are especially important in biology as they are involved in the Krebs citric acid cycle and in glycolysis. Common types of keto acids include: *Alpha-keto acids, alpha-ketoacids, or 2-oxoacids have the keto group adjacent to the carboxylic acid. They often arise by oxidative deamination of amino acids, and reciprocally, they are precursors to the same. Alpha-keto acids possesses extensive chemistry as acylation agents. Furthermore, alpha-keto acids such as phenylpyruvic acid are endogenous sources for carbon monoxide (as a gasotransmitter) and pharmaceutical prodrug scaffold. Important representatives: ** ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |