|
Potassium Fluorosilicate
Potassium fluorosilicate is a chemical compound with the chemical formula . When doped with potassium hexafluoromanganate(IV) (, with ) it forms a narrow band red producing phosphor, (often abbreviated PSF or KSF), of economic interest due to its applicability in LED lighting and displays. Natural occurrence Occurs naturally as hiereatite, found in the Aeolian islands (Sicily, Italy). A hexagonal form demartinite has also been found at the rim of volcanic fumaroles in the same islands. The sea sponge Halichondria Moorei builds a skeleton of potassium fluorosilicate. Structure and properties According to analysis by Loehlin (1984), it has space group Fmm, with a0 = 0.8134 nm, V = 0.538.2 nm3 at 295 K. The Si-F bond length is 0.1683 nm. At high temperatures and pressures -beta and -gamma phases exist. Applications Potassium fluorosilicate has applications in porcelain manufacture, the preservation of timber, aluminium and magnesium smelting, and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ammonium Hexafluorosilicate
Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid.Wiberg, E., Wiberg, N., and Holleman, A. F. (2001) ''Inorganic chemistry''. Academic Press, San Diego. It is made of white crystals, which have at least three polymorphs and appears in nature as rare minerals cryptohalite or bararite. Structure Ammonium fluorosilicate has three major polymorphs: α-(NH4)2 iF6form is cubic (space group Fm3m, No. 225) and corresponds to the mineral cryptohalite. The β form is trigonal (scalenohedral) and occurs in nature as mineral bararite. A third (γ) form was discovered in 2001 and identified with the hexagonal 6mm symmetry. In all three configurations, the iF6sup>2− octahedra are arranged in layers. In the α form, these layers are perpendicular to 11directions. In the β- and γ- forms, the layers are perpendicular to the c-axis. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Lumber
Lumber is wood that has been processed into uniform and useful sizes (dimensional lumber), including beams and planks or boards. Lumber is mainly used for construction framing, as well as finishing (floors, wall panels, window frames). Lumber has many uses beyond home building. Lumber is referred to as timber in the United Kingdom, Australia, and New Zealand, while in other parts of the world, including the United States and Canada, the term ''timber'' refers specifically to unprocessed wood fiber, such as cut logs or standing trees that have yet to be cut. Lumber may be supplied either rough- sawn, or surfaced on one or more of its faces. ''Rough lumber'' is the raw material for furniture-making, and manufacture of other items requiring cutting and shaping. It is available in many species, including hardwoods and softwoods, such as white pine and red pine, because of their low cost. ''Finished lumber'' is supplied in standard sizes, mostly for the construction ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Potassium Compounds
Potassium is a chemical element; it has symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge (which combines with anions to form salts). In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in seawater (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, a common constituent of granites and oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ammonium Fluorosilicate
Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid.Wiberg, E., Wiberg, N., and Holleman, A. F. (2001) ''Inorganic chemistry''. Academic Press, San Diego. It is made of white crystals, which have at least three polymorphs and appears in nature as rare minerals cryptohalite or bararite. Structure Ammonium fluorosilicate has three major polymorphs: α-(NH4)2 iF6form is cubic (space group Fm3m, No. 225) and corresponds to the mineral cryptohalite. The β form is trigonal (scalenohedral) and occurs in nature as mineral bararite. A third (γ) form was discovered in 2001 and identified with the hexagonal 6mm symmetry. In all three configurations, the iF6sup>2− octahedra are arranged in layers. In the α form, these layers are perpendicular to 11directions. In the β- and γ- forms, the layers are perpendicular to the c-axis. ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Potassium Fluoride
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide salt and occurs naturally as the rare mineral carobbiite. Solutions of KF will etch glass due to the formation of soluble fluorosilicates, although HF is more effective. Preparation Potassium fluoride is prepared by reacting potassium carbonate with hydrofluoric acid. Evaporation of the solution forms crystals of potassium bifluoride. The bifluoride on heating yields potassium fluoride: : : Platinum or heat resistant plastic containers are often used for these operations. Potassium chloride converts to KF upon treatment with hydrogen fluoride. In this way, potassium fluoride is recyclable. Crystalline properties KF crystallizes in the cubic NaCl crystal structure. The lattice parameter at room temperature is 0.266 nm. Applications in organic chemistry In organic c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrofluoric Acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling point near room temperature. It is used to make most organofluorine compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers. Uses Production of organofluorine compounds The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon, fluoropolymers, fluorocarbons, and refrigerants such as freon. Many pharmaceuticals contain fluorine. Production of inorganic fluorides Most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
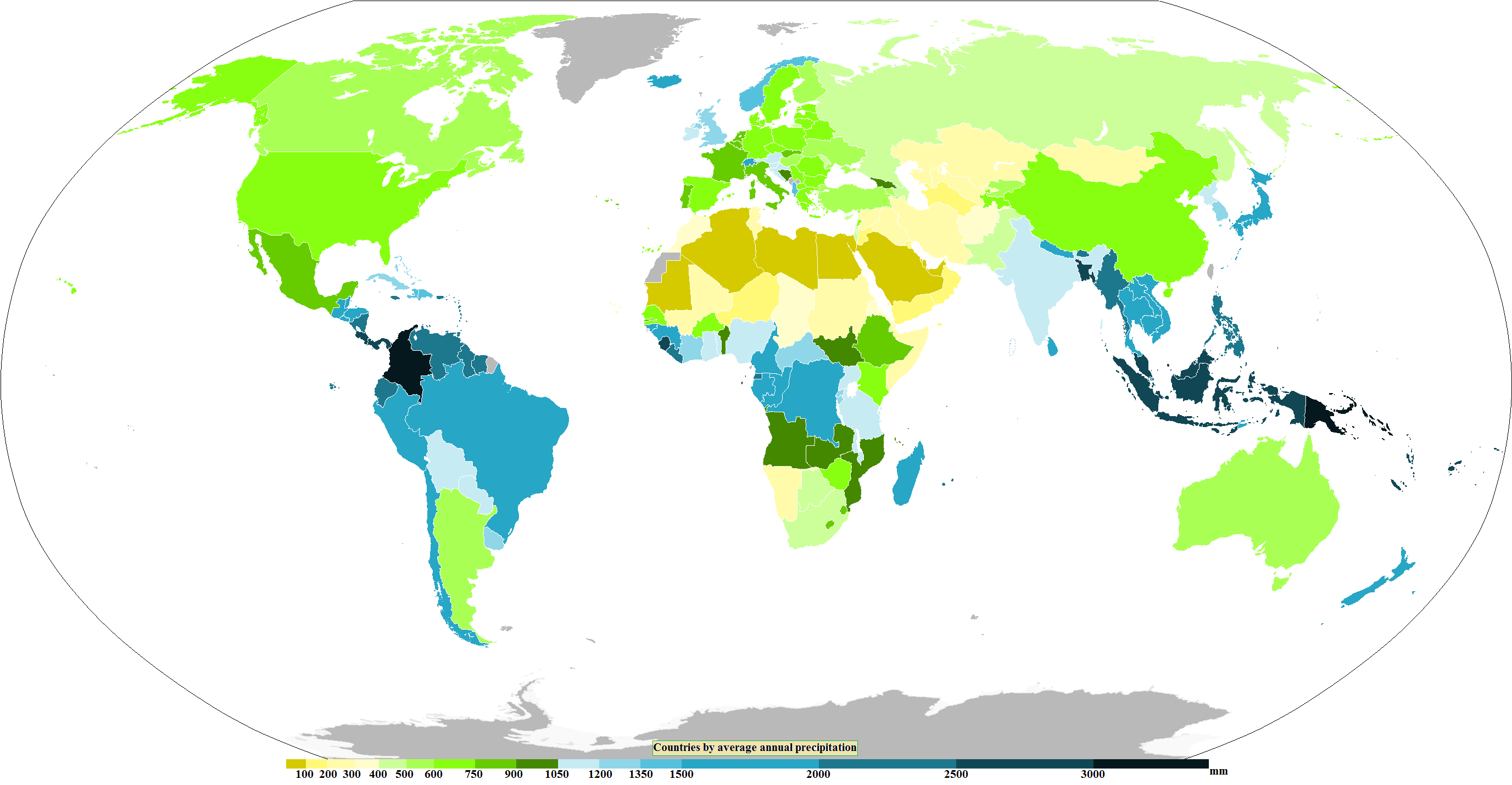
Precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwealth usage), snow, ice pellets, graupel and hail. Precipitation occurs when a portion of the atmosphere becomes saturated with water vapor (reaching 100% relative humidity), so that the water condenses and "precipitates" or falls. Thus, fog and mist are not precipitation; their water vapor does not condense sufficiently to precipitate, so fog and mist do not fall. (Such a non-precipitating combination is a colloid.) Two processes, possibly acting together, can lead to air becoming saturated with water vapor: cooling the air or adding water vapor to the air. Precipitation forms as smaller droplets coalesce via collision with other rain drops or ice crystals within a cloud. Short, intense periods of rain in scattered locations are calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Crystallisation
Crystallization is a process that leads to solids with highly organized atoms or molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regular organization. Crystallization can occur by various routes including precipitation from solution, freezing of a liquid, or deposition from a gas. Attributes of the resulting crystal can depend largely on factors such as temperature, air pressure, cooling rate, or solute concentration. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. Crystallizati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Yttrium Aluminium Garnet
Yttrium aluminium garnet (YAG, Yttrium, Y3Aluminium, Al5Oxygen, O12) is a synthetic crystalline material of the garnet group. It is a Crystal system, cubic yttrium aluminium oxide phase, with other examples being YAlO3 (YAP) in a Crystal system, hexagonal or an orthorhombic, perovskite-like form, and the monoclinic Y4Al2O9 (YAM). Due to its broad optical transparency, low internal stress, high hardness, chemical and heat resistance, YAG is used for a variety of optics. Its lack of birefringence (unlike sapphire) makes it an interesting material for high-energy/high-power laser systems. Laser damage threshold, Laser damage levels of YAG ranged from 1.1 to 2.2 kJ/cm2 (1064 nm, 10 ns). YAG, like garnet and sapphire, has no uses as a laser medium when pure. However, after being doped with an appropriate ion, YAG is commonly used as a host material in various solid-state lasers. Rare earth elements such as neodymium and erbium can be doping (semiconductors), doped into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cerium
Cerium is a chemical element; it has Chemical symbol, symbol Ce and atomic number 58. It is a hardness, soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the oxidation state of +3 characteristic of the series, it also has a stable +4 state that does not oxidize water. It is considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure. Despite always occurring in combination with the other rare-earth elements in minerals such as those of the monazite and bastnäsite groups, cerium is easy to extract from its ores, as it can be distinguished among the lanthanides by its unique ability to be oxidized to the +4 state in aqueous solution. It is the most common of the lanthanides, followed by neodymium, lanthanum, and praseodymium. Its estimated abundance of elements in Earth's crust, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Light-emitting Diode
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresponding to the energy of the photons) is determined by the energy required for electrons to cross the band gap of the semiconductor. White light is obtained by using multiple semiconductors or a layer of light-emitting phosphor on the semiconductor device. Appearing as practical electronic components in 1962, the earliest LEDs emitted low-intensity infrared (IR) light. Infrared LEDs are used in remote-control circuits, such as those used with a wide variety of consumer electronics. The first visible-light LEDs were of low intensity and limited to red. Early LEDs were often used as indicator lamps, replacing small incandescent bulbs, and in seven-segment displays. Later developments produced LEDs available in visible, ultraviolet (U ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
White LED
A light-emitting diode (LED) is a semiconductor device that Light#Light sources, emits light when Electric current, current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresponding to the energy of the photons) is determined by the energy required for electrons to cross the band gap of the semiconductor. White light is obtained by using multiple semiconductors or a layer of light-emitting phosphor on the semiconductor device. Appearing as practical electronic components in 1962, the earliest LEDs emitted low-intensity infrared (IR) light. Infrared LEDs are used in Remote control, remote-control circuits, such as those used with a wide variety of consumer electronics. The first visible-light LEDs were of low intensity and limited to red. Early LEDs were often used as indicator lamps, replacing small Incandescent light bulb, incandescent bulbs, and in seven-segment displays. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |