|
Polyethylene Adipate
Poly(ethylene adipate) or PEA is an aliphatic polyester. It is most commonly synthesized from a polycondensation reaction between ethylene glycol and adipic acid. PEA has been studied as it is biodegradable through a variety of mechanisms and also fairly inexpensive compared to other polymers. Its lower molecular weight compared to many polymers aids in its biodegradability. Synthesis Polycondensation Poly(ethylene adipate) can be synthesized through a variety of methods. First, it could be formed from the polycondensation of dimethyl adipate and ethylene glycol mixed in equal amounts and subjected to increasing temperatures (100 °C, then 150 °C, and finally 180 °C) under nitrogen atmosphere. Methanol is released as a byproduct of this polycondensation reaction and must be distilled off. Second, a melt condensation of ethylene glycol and adipic acid could be carried out at 190-200 °C under nitrogen atmosphere. Lastly, a two-step reaction between adipi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aliphatic Polyester
Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include some naturally occurring chemicals, such as those found in plants and insects. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing. Polyester fibers are sometimes spun together with natural fibers to produce a cloth with blended properties. Cotton-polyester blends can be strong, wrinkle- and tear-resistant, and reduce shrinking. Synthetic fibers using polyester have high water, wind, and environmental resistance compared to plant-derived fibers. They are less Fireproofing, fire-resistant and can melt when ignited. Liquid crystalline polyesters are among the first industrially used liquid crystal polymers. They are used for their mechanical propert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasticizer
A plasticizer ( UK: plasticiser) is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture. Plasticizers are commonly added to polymers and plastics such as PVC, either to facilitate the handling of the raw material during fabrication, or to meet the demands of the end product's application. Plasticizers are especially key to the usability of polyvinyl chloride (PVC), the third most widely used plastic. In the absence of plasticizers, PVC is hard and brittle; with plasticizers, it is suitable for products such as vinyl siding, roofing, vinyl flooring, rain gutters, plumbing, and electric wire insulation/coating. Plasticizers are also often added to concrete formulations to make them more workable and fluid for pouring, thus allowing the water contents to be reduced. Similarly, they are often added to clays, stucco, solid rocket fuel, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
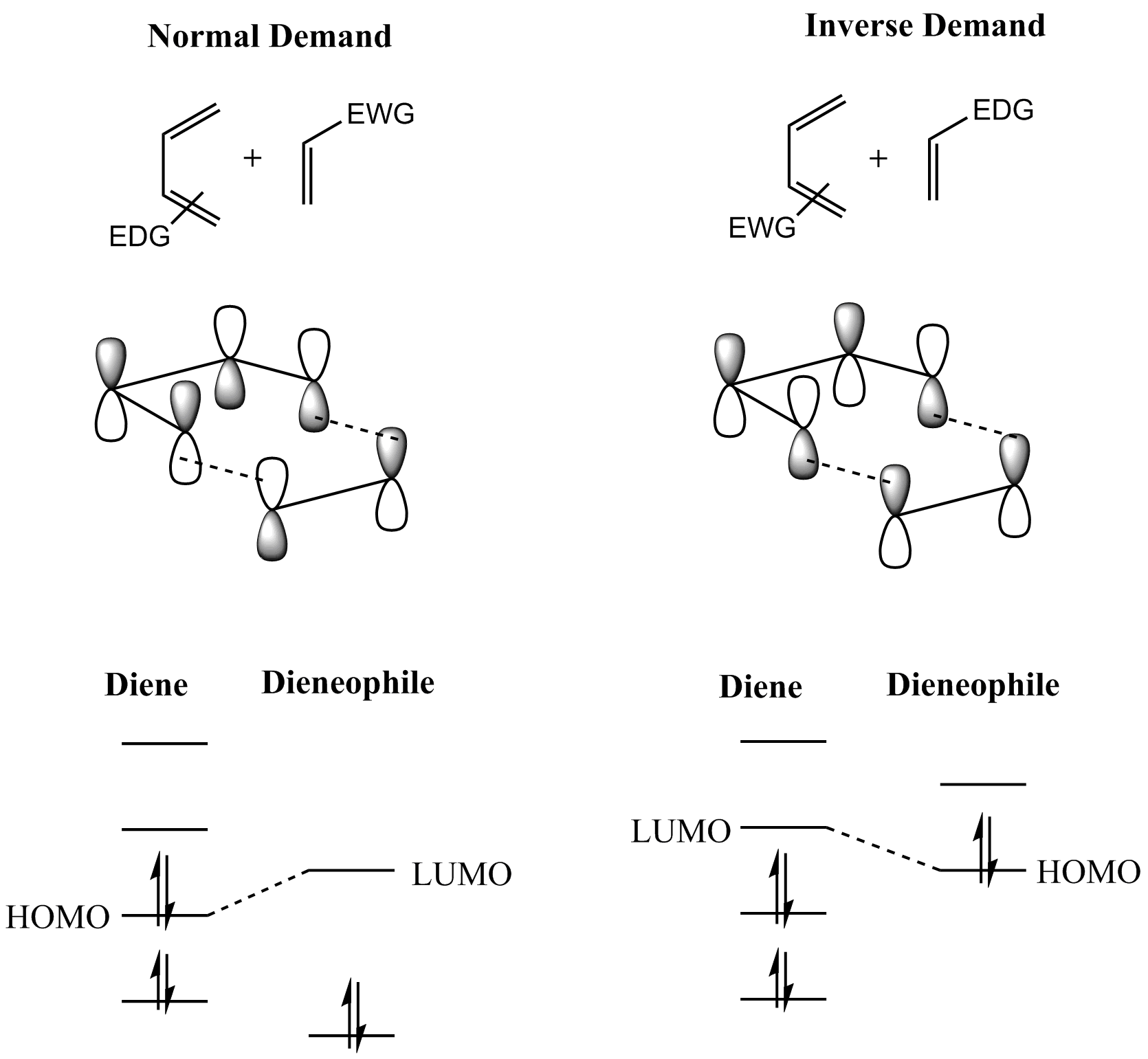
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted reaction, concerted mechanism. More specifically, it is classified as a thermally allowed [4+2] cycloaddition with Woodward–Hoffmann rules, Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brittleness
A material is brittle if, when subjected to stress (physics), stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength of materials, strength. Breaking is often accompanied by a sharp snapping sound. When used in materials science, it is generally applied to materials that fail when there is little or no plasticity (physics), plastic deformation before failure. One proof is to match the broken halves, which should fit exactly since no plastic deformation has occurred. Brittleness in different materials Polymers Mechanical characteristics of polymers can be sensitive to temperature changes near room temperatures. For example, poly(methyl methacrylate) is extremely brittle at temperature 4˚C, but experiences increased ductility with increased temperature. Amorphous polymers are polymers that can behave differently at different temperatures. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...-insoluble liquid with the odor associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group by a single bond. As such, its systematic IUPAC nomenclature of organic chemistry, IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-decanediol
Onekama ( ) is a village in Manistee County in the U.S. state of Michigan. The population was 399 at the 2020 census. The village is located on the northeast shore of Portage Lake and is surrounded by Onekama Township. The town's name is derived from ''Ona-ga-maa'', an Anishinaabe word which means "singing water". History The predecessor of the village of Onekama was the settlement of Portage at Portage Point, first established in 1845, at the western end of Portage Lake, at the outlet of Portage Creek. In 1871, when landowners around the land-locked lake became exasperated with the practices of the Portage Sawmill, they took the solution into their own hands and dug a channel through the narrow isthmus, opening a waterway that lowered the lake by and brought it to the same level as Lake Michigan. When this action dried out Portage Creek on May 14, 1871, the settlement, which had only the week before been designated as "Onekama" with a post office under that name, moved to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-butanediol
1,2-Butanediol is the organic compound with the formula HOCH(HO)CHCHCH. It is classified as a ''vic''-diol (glycol). It is chiral, although typically it is encountered as the racemic mixture. It is a colorless liquid. Preparation This diol was first described by Charles-Adolphe Wurtz in 1859. It is produced industrially by hydration of 1,2-epoxybutane.. : This process requires a ten- to twenty-fold excess of water to suppress the formation of polyethers. Depending on the amount of excess water, the selectivity varies from 70 to 92%. Sulfuric acid or strongly acidic ion exchange resins may be used as catalysts, which allows the reaction to occur under 160 °C and at slightly above atmospheric pressure. 1,2-Butanediol is a byproduct of the production of 1,4-butanediol from butadiene.. It is also a byproduct of the catalytic hydrocracking of starches and sugars such as sorbitol to ethylene glycol and propylene glycol. It can also be obtained from the dihydroxylation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic Effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and to be excluded by water. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar substances, which maximizes the entropy of water and minimizes the area of contact between water and nonpolar molecules. In terms of thermodynamics, the hydrophobic effect is the free energy change of water surrounding a solute. A positive free energy change of the surrounding solvent indicates hydrophobicity, whereas a negative free energy change implies hydrophilicity. The hydrophobic effect is responsible for the separation of a mixture of oil and water into its two components. It is also responsible for effects related to biology, including: cell membrane and vesicle formation, protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations. Hence the hydrophobic effect i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esterase
In biochemistry, an esterase is a class of enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis (and as such, it is a type of hydrolase). A wide range of different esterases exist that differ in their substrate specificity, their protein structure, and their biological function. EC classification/list of enzymes * ''EC 3.1.1'': Carboxylic ester hydrolases ** Acetylesterase (EC 3.1.1.6), splits off acetyl groups *** Cholinesterase **** Acetylcholinesterase, inactivates the neurotransmitter acetylcholine **** Pseudocholinesterase, broad substrate specificity, found in the blood plasma and in the liver ** Pectinesterase (EC 3.1.1.11), clarifies fruit juices * ''EC 3.1.2'': Thiolester hydrolases ** Thioesterase *** Ubiquitin carboxy-terminal hydrolase L1 * ''EC 3.1.3'': Phosphoric monoester hydrolases ** Phosphatase (EC 3.1.3.x), hydrolyses phosphoric acid monoesters into a phosphate ion and an alcohol *** Alkaline phosp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipase
In biochemistry, lipase ( ) refers to a class of enzymes that catalyzes the hydrolysis of fats. Some lipases display broad substrate scope including esters of cholesterol, phospholipids, and of lipid-soluble vitamins and sphingomyelinases; however, these are usually treated separately from "conventional" lipases. Unlike esterases, which function in water, lipases "are activated only when adsorbed to an oil–water interface". Lipases perform essential roles in digestion, transport and processing of dietary lipids in most, if not all, organisms. Structure and catalytic mechanism Classically, lipases catalyse the hydrolysis of triglycerides: \begin \text + \ce &\longrightarrow \text + \text \\[4pt] \text + \ce &\longrightarrow \text + \text \\[4pt] \text + \ce &\longrightarrow \text + \text \end Lipases are serine hydrolases, i.e. they function by transesterification generating an acyl serine intermediate. Most lipases act at a specific position on the glycerol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low-density Polyethylene
Low-density polyethylene (LDPE) is a thermoplastic made from the monomer ethylene. It was the first grade of polyethylene, produced in 1933 by John C. Swallow and M.W Perrin who were working for Imperial Chemical Industries (ICI) using a high pressure process via free radical polymerization. Its manufacture employs the same method today. The EPA estimates 5.7% of LDPE ( resin identification code 4) is recycled in the United States. Despite competition from more modern polymers, LDPE continues to be an important plastic grade. In 2013 the worldwide LDPE market reached a volume of about US$33 billion. Despite its designation with the recycling symbol, it cannot be as commonly recycled as No. 1 (polyethylene terephthalate) or 2 plastics (high-density polyethylene). Properties LDPE is defined by a density range of 917–930 kg/m3. At room temperature it is not reactive, except to strong oxidizers; some solvents cause it to swell. It can withstand temperatures of continuou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polybutylene Succinate
Polybutylene succinate (PBS) (sometimes written polytetramethylene succinate) is a thermoplastic polymer resin of the polyester family. PBS is a biodegradable aliphatic polyester with properties that are comparable to polypropylene. It may also be referred to by the brand names GsPLA or BioPBS (Mitsubishi Chemical). PBS consists of polymerized units of butylene succinate, with repeating units. History The synthesis of succinic acid based polyesters was first performed in 1863. In that time the Portuguese professor Agostinho Vicente Lourenço described in his "Recherche sur les composés polyatomiques" (Research on polyatomic compounds), the reaction between succinic acid and ethylene glycol to form what he named "succino-ethylenic acid". He noticed that this acid was losing water when it was heated at high temperatures (300 °C) and that a crystalline mass when obtained after cooling. Unfortunately, Lourenço did not study much the structure of the material he obtained ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |