|
Phosphorus Trioxide
Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to adamantane. It is formally the anhydride of phosphorous acid, H3PO3, but cannot be obtained by the dehydration of the acid. A white solid that melts at room temperature, it is waxy, crystalline and highly toxic, with garlic odor. Preparation It is obtained by the combustion of phosphorus in a limited supply of air at low temperatures. :P4 + 3 O2 → P4O6 By-products include red phosphorus suboxide. Chemical properties Phosphorus trioxide reacts with water to form phosphorous acid, reflecting the fact that it is the anhydride of that acid. : P4O6 + 6 H2O → 4 H3PO3 It reacts with hydrogen chloride to form H3PO3 and phosphorus trichloride. : P4O6 + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Sesquioxide
A sesquioxide is an oxide of an element (or radical), where the ratio between the number of atoms of that element and the number of atoms of oxygen is 2:3. For example, aluminium oxide and phosphorus(III) oxide are sesquioxides. Many sesquioxides contain a metal in the +3 oxidation state and the oxide ion , e.g., aluminium oxide , lanthanum(III) oxide and iron(III) oxide . Sesquioxides of iron and aluminium are found in soil. The alkali metal sesquioxides are exceptions because they contain both peroxide and superoxide ions, e.g., rubidium sesquioxide is formulated . Sesquioxides of metalloids and nonmetals are better formulated as covalent, e.g. boron trioxide , dinitrogen trioxide and phosphorus(III) oxide ; chlorine trioxide and bromine trioxide do not have oxidation state +3 on the halogen. Many transition metal oxides crystallize in the corundum structure type, with space group Rc. Sesquioxides of rare earth elements crystalize into one or more of three crystal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Diphosphorus Tetraiodide
Diphosphorus tetraiodide is an orange crystalline solid with the formula >. It has been used as a reducing agent in organic chemistry. It is a rare example of a compound with phosphorus in the +2 oxidation state, and can be classified as a subhalide of phosphorus. It is the most stable of the diphosphorus tetrahalides. Synthesis and structure Diphosphorus tetraiodide is easily generated by the disproportionation of phosphorus triiodide in dry ether: : It can also be obtained by treating phosphorus trichloride and potassium iodide in anhydrous conditions. Another synthesis route involves combining phosphonium iodide with iodine in a solution of carbon disulfide. An advantage of this route is that the resulting product is virtually free of impurities. : The compound adopts a centrosymmetric structure with a P-P bond of 2.230 Å. Reactions Inorganic chemistry Diphosphorus tetraiodide reacts with bromine to form mixtures . With sulfur, it is oxidized to , retaining the P-P bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
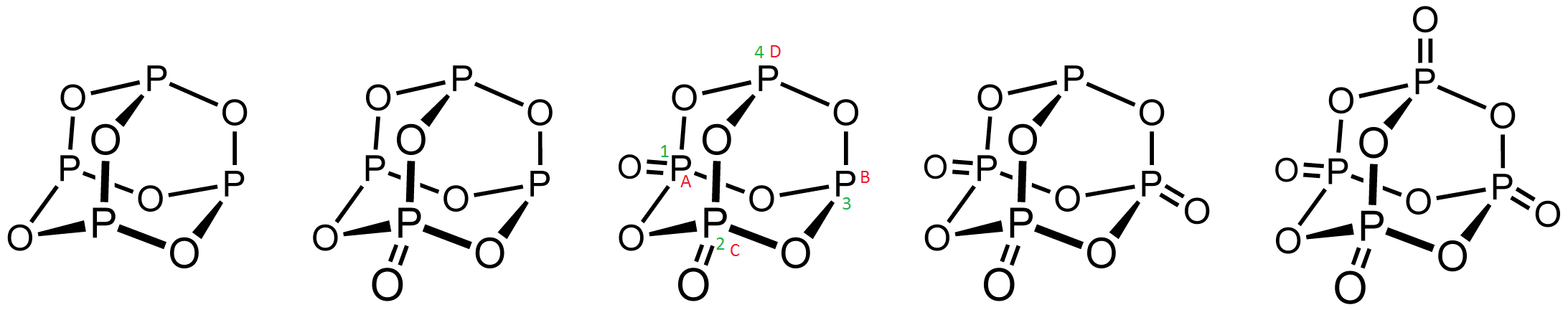
Phosphorus Oxides
Phosphorus oxide can refer to: * Phosphorus pentoxide (phosphorus(V) oxide, phosphoric anhydride), P2O5 * Phosphorus trioxide (phosphorus(III) oxide, phosphorous anhydride), P2O3 * Phosphorus tetroxide, Between the commercially important P4O6 and P4O10, several other, less common oxides of phosphorus are known. Specifically, P4O7, P4O9, and P2O6 all bear structures intermediate between the endmembers:Luer, B.; Jansen, M. "Crystal Structure Refinement of Tetraphosphorus Nonaoxide, P4O9" Zeitschrift für Kristallographie 1991, volume 197, pages 247-8. On observation it will be seen that double bonded oxygen in P4O8 at 1,2 position or 1,3 position are identical and both positions have same steric hindrance. Cycle 12341 and ABCDA are identical. Gases: * Phosphorus monoxide Phosphorus monoxide is an unstable radical inorganic compound with molecular formula P O. Phosphorus monoxide is notable as one of the few molecular compounds containing phosphorus that has been detected out ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Sesquioxides
A sesquioxide is an oxide of an element (or radical), where the ratio between the number of atoms of that element and the number of atoms of oxygen is 2:3. For example, aluminium oxide and phosphorus(III) oxide are sesquioxides. Many sesquioxides contain a metal in the +3 oxidation state and the oxide ion , e.g., aluminium oxide , lanthanum(III) oxide and iron(III) oxide . Sesquioxides of iron and aluminium are found in soil. The alkali metal sesquioxides are exceptions because they contain both peroxide and superoxide ions, e.g., rubidium sesquioxide is formulated . Sesquioxides of metalloids and nonmetals are better formulated as covalent, e.g. boron trioxide , dinitrogen trioxide and phosphorus(III) oxide ; chlorine trioxide and bromine trioxide do not have oxidation state +3 on the halogen. Many transition metal oxides crystallize in the corundum structure type, with space group Rc. Sesquioxides of rare earth elements crystalize into one or more of three crystal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Adduct Of Phosphorus Oxide With BH3
In chemistry, an adduct (; alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species. Examples include the addition of sodium bisulfite to an aldehyde In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ... to give a sulfonate. It can be considered as a single product resulting from the direct combination of different molecules which comprises all atoms of the reactant molecules. Adducts often form between Lewis acids and Lewis bases. A good example is the formation of adducts between the Lewis acid borane and the oxygen atom in the Lewis bases, tetrahydrofuran (THF): or diethyl ether: . M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Borane
Borane is an inorganic compound with the chemical formula . Because it tends to dimerize or form adducts, borane is very rarely observed. It normally dimerizes to diborane in the absence of other chemicals. It can be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. Structure and properties BH3 is a trigonal planar molecule with D3h symmetry. The experimentally determined B–H bond length is 119 pm. In the absence of other bases, it dimerizes to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction: :BX3 +BH4− → HBX3− + (BH3) (X=F, Cl, Br, I) :2 BH3 → B2H6 The standard enthalpy of dimerization of BH3 is estimated to be −170 kJ mol−1. The boron atom in BH3 has 6 valence electrons. Consequently, it is a strong Lewis acid and reacts with any Lewis base ('L' in equation below) to form an adduct: :BH3 + L � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phosphite
The general structure of a phosphite ester showing the lone pairs on the P In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids. Synthesis ;From PCl3 Phosphite esters are typically prepared by treating phosphorus trichloride with an alcohol. For alkyl alcohols the displaced chloride ion can attack the phosphite, causing dealkylation to give a dialkylphosphite and an organochlorine compound. The overall reaction is as follows: :PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl Alternatively, when the alcoh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Disproportionation Reaction
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called ''comproportionation'', also known as ''symproportionation''. More generally, the term can be applied to any desymmetrizing reaction where two molecules of one type react to give one each of two different types: : This expanded definition is not limited to redox reactions, but also includes some molecular autoionization reactions, such as the self-ionization of water. In contrast, some authors use the term ''redistribution'' to refer to reactions of this type (in either direction) when only ligand exchange but no redox is involved and distinguish such processes from disproportionation and comproportionati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Reaction Of Phosphoprus Trioxide With Ozone
Reaction may refer to a process or to a response to an action, event, or exposure. Physics and chemistry *Chemical reaction *Nuclear reaction *Reaction (physics), as defined by Newton's third law *Chain reaction (other) Biology and medicine *Adverse drug reaction *Allergic reaction *Reflex, neural reaction *Hypersensitivity, immune reaction *Intolerance (other) *Light reaction (other) Psychology *Emotional, reaction * Reactivity *Proactivity, opposite of reactive behaviour *Reactive attachment disorder Politics and culture *Reactionary, a political tendency *Reaction video *Commentary (other) Proper names and titles * ''Reaction'' (album), a 1986 album by American R&B singer Rebbie Jackson ** "Reaction" (song), the title song from the Rebbie Jackson album *"Reaction", a single by Dead Letter Circus *''Reactions'', a 2018 album by The Mods *ReAction GUI, a GUI toolkit used on AmigaOS * Reaction.life, a political news and commentary websi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odor is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 chemical structure, structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetism, diamagnetic. At standard temperature and pressure, ozone is a pale blue gas that condenses at cryogenic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |