|
Pentaborane(9)
Pentaborane(9) is an inorganic compound with the formula . It is one of the most common boron hydride clusters, although it is a highly reactive compound. Because of its high reactivity with oxygen, it was once evaluated as rocket or jet fuel. Like many of the smaller boron hydrides, pentaborane is colourless, diamagnetic, and volatile. It is related to pentaborane(11) (). Structure, synthesis, properties Its structure is that of five atoms of boron arranged in a square pyramid. Each boron has a terminal hydride ligand and four hydrides span the edges of the base of the pyramid. It is classified as a nido cage. It was first prepared by Alfred Stock by pyrolysis of diborane at about 200 °C. An improved synthesis starts from salts of octahydrotriborate (), which is converted to the bromide using HBr. Pyrolysis of this bromide gives pentaborane. : In the U.S., pentaborane was produced on a commercial scale by Callery Chemical Company. Above 150 °C, it decomposes, pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Boron Hydride Cluster
Boron hydride clusters are compounds with the formula or related anions, where x ≥ 3. Many such cluster compounds are known. Common examples are those with 5, 10, and 12 boron atoms. Although they have few practical applications, the borane hydride clusters exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed. History The development of the borane hydride clusters resulted from pioneering work by Alfred Stock, invented the glass vacuum line for their study. The structures of the boron hydride clusters were determined beginning in 1948 with the characterization of decaborane. William Lipscomb was awarded the Nobel Prize in Chemistry in 1976 for this and many subsequent crystallographic investigations. These investigations revealed the prevalence of deltahedral structures, i.e., networks of triangular arrays of BH centers. The bonding of the clusters ushered in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Octahydrotriborate
Octahydrotriborate is the boron hydride anion B3H8−. It forms a variety of salts that are colorless and air-stable. The tetrabutylammonium salt is soluble in organic solvents such as acetonitrile and methylene chloride. The anion is an intermediate is the synthesis of various higher boron hydrides, such as pentaborane(9). B3H8− can be viewed as the conjugate base of triborane B3H9. Preparation Octahydrotriborate is prepared by partial oxidation of borohydride with iodine or boron trifluoride: :3BH4− + I2 → B3H8− + 2H2 + 2I− :5BH4− + 4BF3O(C2H5)2 → 2B3H8− + 2H2 + 4O(C2H5)2 + 3BF4− Structure and reactions As shown by X-ray crystallography of various salts, B3H8− consists of a distorted triangle of three BH2 vertices. Two edges of the triangle are occupied by bridging hydrides. It is converted to the bromide B3H7Br− using HBr (illustrating its hydridic character): :B3H8− + HBr → B3H7Br− + H2 Pyrolysis of this bromide gives p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Diborane
Diborane(6), commonly known as diborane, is the chemical compound with the formula . It is a highly toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents. Structure and bonding The structure of diborane has D2h symmetry. Four hydrides are terminal, while two bridge between the boron centers. The lengths of the B–Hbridge bonds and the B–Hterminal bonds are 1.33 and 1.19 Å respectively. This difference in bond lengths reflects the difference in their strengths, the B–Hbridge bonds being relatively weaker. The weakness of the B–Hbridge compared to B–Hterminal bonds is indicated by their vibrational signatures in the infrared spectrum, being ≈2100 and 2500 cm−1 respectively. The model determined by molecular orbital theory describes the bonds between boron and the te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovas and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is found in smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
WayBack Machine
The Wayback Machine is a digital archive of the World Wide Web founded by Internet Archive, an American nonprofit organization based in San Francisco, California. Launched for public access in 2001, the service allows users to go "back in time" to see how websites looked in the past. Founders Brewster Kahle and Bruce Gilliat developed the Wayback Machine to provide "universal access to all knowledge" by preserving archived copies of defunct web pages. The Wayback Machine's earliest archives go back at least to 1995, and by the end of 2009, more than 38.2 billion webpages had been saved. As of November 2024, the Wayback Machine has archived more than 916 billion web pages and well over 100 petabytes of data. History The Internet Archive has been archiving cached web pages since at least 1995. One of the earliest known pages was archived on May 8, 1995. Internet Archive founders Brewster Kahle and Bruce Gilliat launched the Wayback Machine in San Francisco, California ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
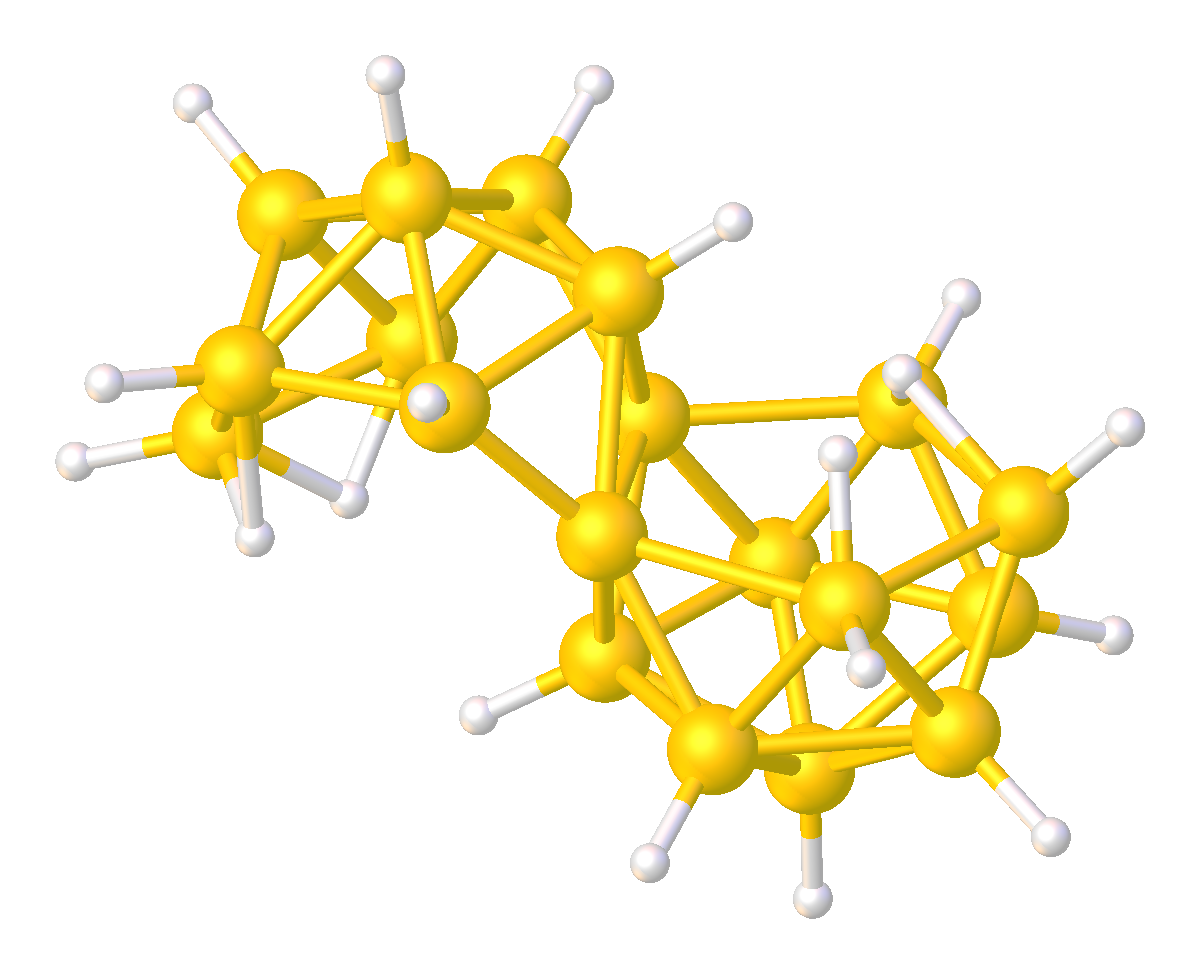
Metallaborane
In chemistry, a metalloborane is a compound that contains one or more metal atoms and one or more boron hydride. These compounds are related conceptually and often synthetically to the boranes, boron-hydride clusters by replacement of BHn units with metal-containing fragments. Often these metal fragments are derived from metal carbonyls or cyclopentadienyl complexes. Their structures can often be rationalized by polyhedral skeletal electron pair theory. The inventory of these compounds is large, and their structures can be quite complex. Examples Two simple examples are . The MB4 cores (M = Fe or Co) of these two compounds adopt structures expected for nido 5-vertex clusters. The iron compound is produced by reaction of diiron nonacarbonyl with pentaborane. and cyclobutadieneiron tricarbonyl have similar structures. Metallacarboranes Even greater in scope than metalloboranes are metallacarboranes. These cages have carbon vertices, often CH, in addition to BH and M vert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Lewis Acids And Bases
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane CH3)3Bis a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Trimethylphosphine
Trimethylphosphine is an organophosphorus compound with the formula P(CH3)3, commonly abbreviated as PMe3. This colorless liquid has a strongly unpleasant odor, characteristic of alkylphosphines. The compound is a common ligand in coordination chemistry. Structure and bonding It is a pyramidal molecule with approximate ''C''3''v'' point group, symmetry. The C–P–C bond angles are approximately 98.6°. The C–P–C bond angles are consistent with the notion that phosphorus predominantly uses the 3p orbitals for forming bonds and that there is little sp hybridization of the phosphorus atom. The latter is a common feature of the chemistry of phosphorus. As a result, the lone pair of trimethylphosphine has predominantly s-character as is the case for phosphine, PH3. PMe3 can be prepared by the treatment of triphenyl phosphite with methylmagnesium chloride: : 3 CH3MgCl + P(OC6H5)3 → P(CH3)3 + 3 C6H5OMgCl The synthesis is conducted in dibutyl ether, from which the more volatil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Heat Of Combustion
The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of heat released during the combustion of a specified amount of it. The ''calorific value'' is the total energy released as heat when a substance undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon or other organic molecule reacting with oxygen to form carbon dioxide and water and release heat. It may be expressed with the quantities: * energy/ mole of fuel * energy/mass of fuel * energy/volume of the fuel There are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much the products are allowed to cool and whether compounds like are allowed to condense. The high heat values are conventionally measured with a bomb calorimeter. Low heat values are calculated from high heat value test data. They may also be calculated as the difference be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Diiron Nonacarbonyl
Diiron nonacarbonyl is an organometallic compound with the formula Fe2(CO)9. This metal carbonyl is a reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe(0) than Fe(CO)5. This micaceous orange solid is virtually insoluble in all common solvents. Synthesis and structure Following the original method, photolysis of an acetic acid solution of Fe(CO)5 produces Fe2(CO)9 in good yield:King, R. B. Organometallic Syntheses. Volume 1 Transition-Metal Compounds; Academic Press: New York, 1965. . :2 Fe(CO)5 → Fe2(CO)9 + CO Fe2(CO)9 consists of a pair of Fe(CO)3 centers linked by three bridging CO ligands. Although older textbooks show an Fe-Fe bond consistent with the 18 electron rule (8 valence electrons from Fe, two each from the terminal carbonyls, one each from the bridging carbonyls and one from the other Fe atom in the metal-metal bond), theoretical analyses have consistently indicated the absence of a direct Fe-F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Grease (lubricant)
Grease is a solid or semisolid lubricant formed as a dispersion of thickening agents in a liquid lubricant. Grease generally consists of a soap emulsified with mineral or vegetable oil. A common feature of greases is that they possess high initial viscosities, which upon the application of shear, drop to give the effect of an oil-lubricated bearing of approximately the same viscosity as the base oil used in the grease. This change in viscosity is called shear thinning. Grease is sometimes used to describe lubricating materials that are simply soft solids or high viscosity liquids, but these materials do not exhibit the shear-thinning properties characteristic of the classical grease. For example, petroleum jellies such as Vaseline are not generally classified as greases. Greases are applied to mechanisms that can be lubricated only infrequently and where a lubricating oil would not stay in position. They also act as sealants to prevent the ingress of water and incompressible ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |