|
P-orbitals
In quantum mechanics, an atomic orbital () is a function describing the location and wave-like behavior of an electron in an atom. This function describes an electron's charge distribution around the atom's nucleus, and can be used to calculate the probability of finding an electron in a specific region around the nucleus. Each orbital in an atom is characterized by a set of values of three quantum numbers , , and , which respectively correspond to electron's energy, its orbital angular momentum, and its orbital angular momentum projected along a chosen axis (magnetic quantum number). The orbitals with a well-defined magnetic quantum number are generally complex-valued. Real-valued orbitals can be formed as linear combinations of and orbitals, and are often labeled using associated harmonic polynomials (e.g., ''xy'', ) which describe their angular structure. An orbital can be occupied by a maximum of two electrons, each with its own projection of spin m_s. The simple n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is , meaning that the 1s, 2s, and 2p subshells are occupied by two, two, and six electrons, respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by the nuclei and all the other electrons. Mathematically, configurations are described by Slater determinants or configuration state functions. According to the laws of quantum mechanics, a level of energy is associated with each electron configuration. In certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understanding the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute Group (periodic table)#Group names, group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of periodic trends, group trends in properties in the periodic table, with elements exhibiting well-characterised Homologous series, homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all shiny, hardness, sof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neon Orbitals
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of air. Neon was discovered in 1898 alongside krypton and xenon, identified as one of the three remaining rare inert elements in dry air after the removal of nitrogen, oxygen, argon, and carbon dioxide. Its discovery was marked by the distinctive bright red emission spectrum it exhibited, leading to its immediate recognition as a new element. The name ''neon'' originates from the Greek word , a neuter singular form of (), meaning 'new'. Neon is a chemically inert gas; although neon compounds do exist, they are primarily ionic molecules or fragile molecules held together by van der Waals forces. The synthesis of most neon in the cosmos resulted from the nuclear fusion within stars of oxygen and helium through the alpha-capture process. Des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Principal Series (spectroscopy)
In atomic emission spectroscopy, the principal series is a series of spectral lines caused when electrons move between p orbitals of an atom and the lowest available s orbital. These lines are usually found in the visible and ultraviolet portions of the electromagnetic spectrum. The principal series has given the letter ''p'' to the p atomic orbital and subshell. The lines are absorption lines when the electron gains energy from an s subshell to a p subshell. When electrons descend in energy they produce an emission spectrum. The term principal came about because this series of lines is observed both in absorption and emission for alkali metal vapours. Other series of lines appear in the emission spectrum only and not in the absorption spectrum, and were named the sharp series and the diffuse series The diffuse series is a series of spectral lines in the atomic emission spectrum caused when electrons jump between the lowest p orbital and d orbitals of an atom. The total orbita ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Harmonics
In physics, acoustics, and telecommunications, a harmonic is a sinusoidal wave with a frequency that is a positive integer multiple of the ''fundamental frequency'' of a periodic signal. The fundamental frequency is also called the ''1st harmonic''; the other harmonics are known as ''higher harmonics''. As all harmonics are periodic at the fundamental frequency, the sum of harmonics is also periodic at that frequency. The set of harmonics forms a '' harmonic series''. The term is employed in various disciplines, including music, physics, acoustics, electronic power transmission, radio technology, and other fields. For example, if the fundamental frequency is 50 Hz, a common AC power supply frequency, the frequencies of the first three higher harmonics are 100 Hz (2nd harmonic), 150 Hz (3rd harmonic), 200 Hz (4th harmonic) and any addition of waves with these frequencies is periodic at 50 Hz. In music, harmonics are used on string instruments and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fundamental Frequency
The fundamental frequency, often referred to simply as the ''fundamental'' (abbreviated as 0 or 1 ), is defined as the lowest frequency of a Periodic signal, periodic waveform. In music, the fundamental is the musical pitch (music), pitch of a note that is perceived as the lowest Harmonic series (music)#Partial, partial present. In terms of a superposition of Sine wave, sinusoids, the fundamental frequency is the lowest frequency sinusoidal in the sum of harmonically related frequencies, or the frequency of the difference between adjacent frequencies. In some contexts, the fundamental is usually abbreviated as 0, indicating the lowest frequency Zero-based numbering, counting from zero. In other contexts, it is more common to abbreviate it as 1, the first harmonic. (The second harmonic is then 2 = 2⋅1, etc.) According to Benward and Saker's ''Music: In Theory and Practice'': Explanation All sinusoidal and many non-sinusoidal waveforms repeat exactly over time – they are per ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
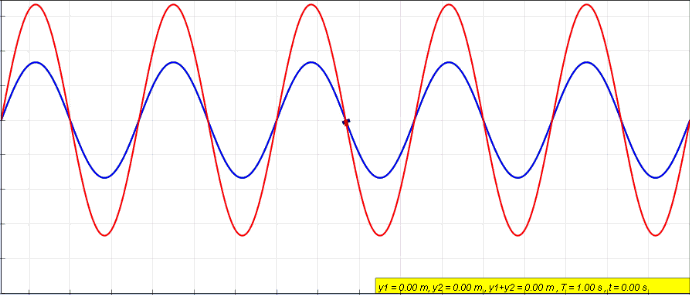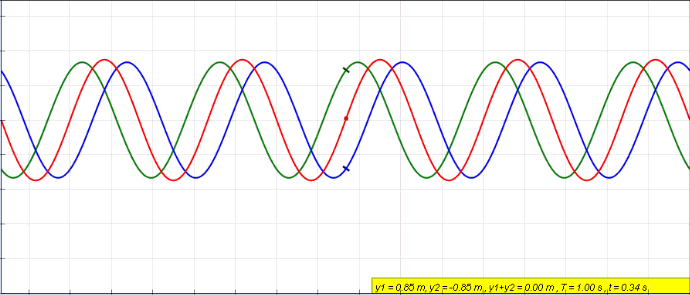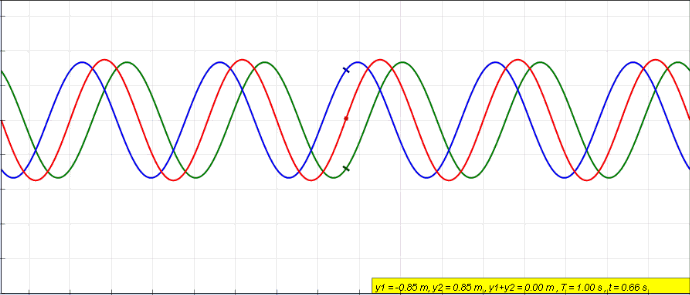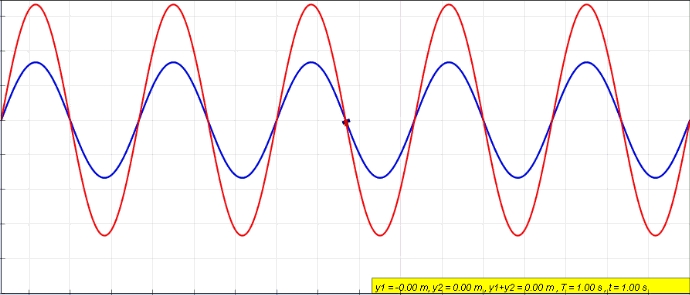
Standing Wave
In physics, a standing wave, also known as a stationary wave, is a wave that oscillates in time but whose peak amplitude profile does not move in space. The peak amplitude of the wave oscillations at any point in space is constant with respect to time, and the oscillations at different points throughout the wave are in phase. The locations at which the absolute value of the amplitude is minimum are called node (physics), nodes, and the locations where the absolute value of the amplitude is maximum are called antinodes. Standing waves were first described scientifically by Michael Faraday in 1831. Faraday observed Faraday wave, standing waves on the surface of a liquid in a vibrating container. Franz Melde coined the term "standing wave" (German: ''stehende Welle'' or ''Stehwelle'') around 1860 and demonstrated the phenomenon in his classic experiment with vibrating strings. This phenomenon can occur because the medium is moving in the direction opposite to the movement of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wave–particle Duality
Wave–particle duality is the concept in quantum mechanics that fundamental entities of the universe, like photons and electrons, exhibit particle or wave (physics), wave properties according to the experimental circumstances. It expresses the inability of the Classical physics, classical concepts such as particle or wave to fully describe the behavior of quantum objects. During the 19th and early 20th centuries, light was found to behave as a wave then later was discovered to have a particle-like behavior, whereas electrons behaved like particles in early experiments then were later discovered to have wave-like behavior. The concept of duality arose to name these seeming contradictions. History Wave-particle duality of light In the late 17th century, Sir Isaac Newton had advocated that light was Corpuscular theory of light, corpuscular (particulate), but Christiaan Huygens took an opposing wave description. While Newton had favored a particle approach, he was the first to at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Density Plots
Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest and most abundant chemical element in the universe, constituting about 75% of all normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovered its property of producing water when burned; hence its name means ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal. Chromium is valued for its high corrosion resistance and hardness. A major development in steel production was the discovery that steel could be made highly resistant to corrosion and discoloration by adding metallic chromium to form stainless steel. Stainless steel and chrome plating (electroplating with chromium) together comprise 85% of the commercial use. Chromium is also greatly valued as a metal that is able to be highly polishing, polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, and almost 90% of infrared, infrared light. The name of the element is derived from the Ancient Greek, Greek word χρῶμα, ''chrōma'', meaning color, because many chromium compounds are intensely colored. Indust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aufbau Principle
In atomic physics and quantum chemistry, the Aufbau principle (, from ), also called the Aufbau rule, states that in the ground state of an atom or ion, electrons first fill Electron shell#Subshells, subshells of the lowest available energy, then fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible. An example is the configuration for the phosphorus atom, meaning that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, and so on. The configuration is often abbreviated by writing only the valence electrons explicitly, while the core electrons are replaced by the symbol for the last previous noble gas in the periodic table, placed in square brackets. For phosphorus, the last previous noble gas is neon, so the configuration is abbreviated to [Ne] 3s2 3p3, where [Ne] signifies the core ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
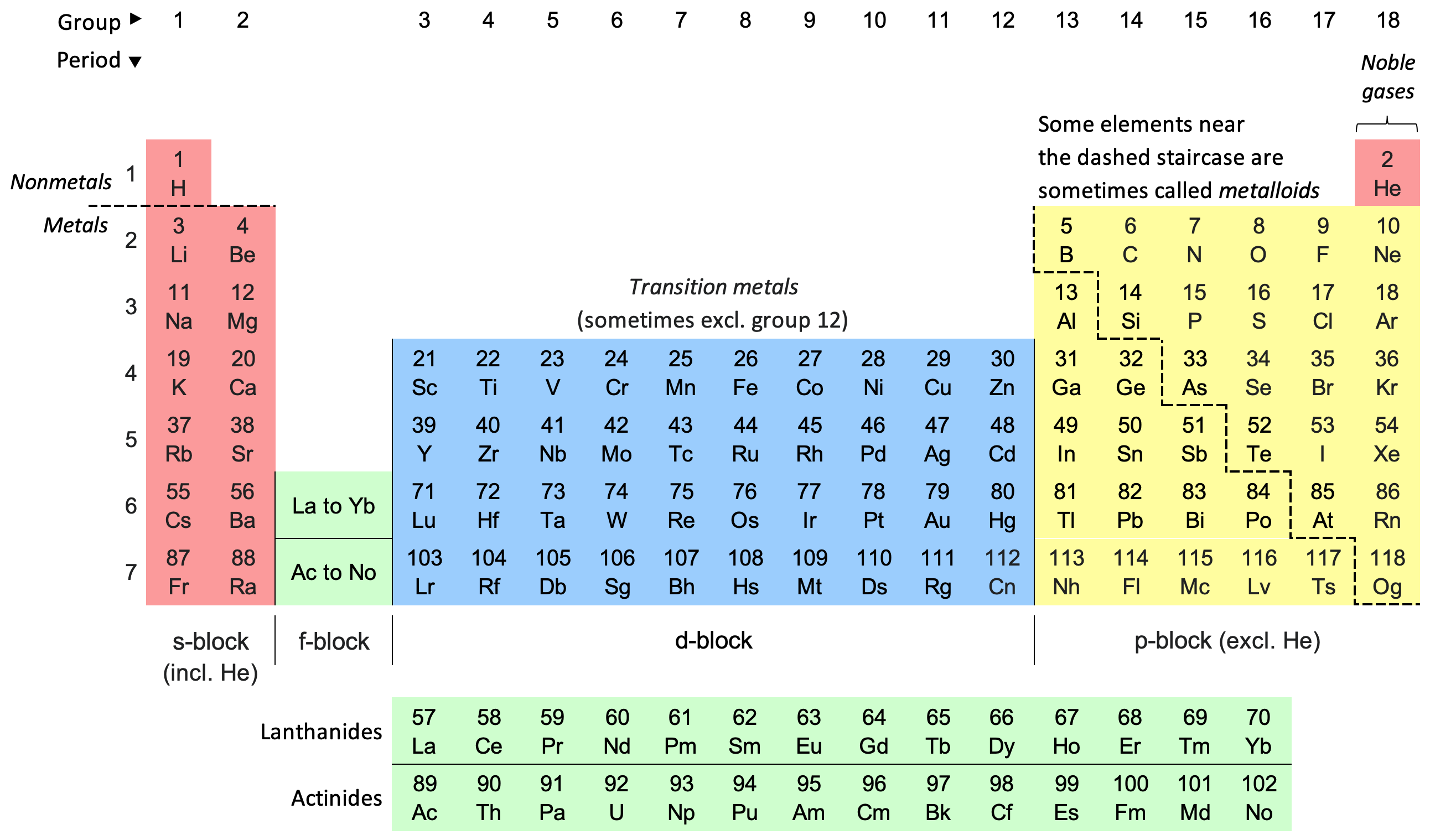
Periodic Table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics. Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |