|
Oxygen Balance
Oxygen balance (OB, OB%, or Ω) is an expression that is used to indicate the degree to which an explosive can be oxidized, to determine whether the molecules of explosive substance or mixture contains enough oxygen to fully oxidize the other atoms in the molecules. For example, fully oxidized carbon forms carbon dioxide, hydrogen forms water, sulfur forms sulfur dioxide, and metals form metal oxides. A molecule is said to have a positive oxygen balance if it contains more oxygen than is needed and a negative oxygen balance if it contains less oxygen than is needed. An explosive with a negative oxygen balance will lead to incomplete combustion, which commonly produces carbon monoxide, which is a toxic gas. Explosives with negative or positive oxygen balance are commonly mixed with other energetic materials that are either oxygen positive or negative, respectively, to increase the explosive's power. For example, TNT is an oxygen negative explosive and is commonly mixed with oxygen p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Explosive
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances. The potential energy stored in an explosive material may, for example, be: * chemical energy, such as nitroglycerin or grain dust * pressurized gas, such as a gas cylinder, aerosol can, or boiling liquid expanding vapor explosion * nuclear energy, such as in the fissile isotopes uranium-235 and plutonium-239 Explosive materials may be categorized by the speed at which they expand. Materials that detonate (the front of the chemical reaction moves faster through the material than the speed of sound) are said to be "high explosives" and materials that deflagrate ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
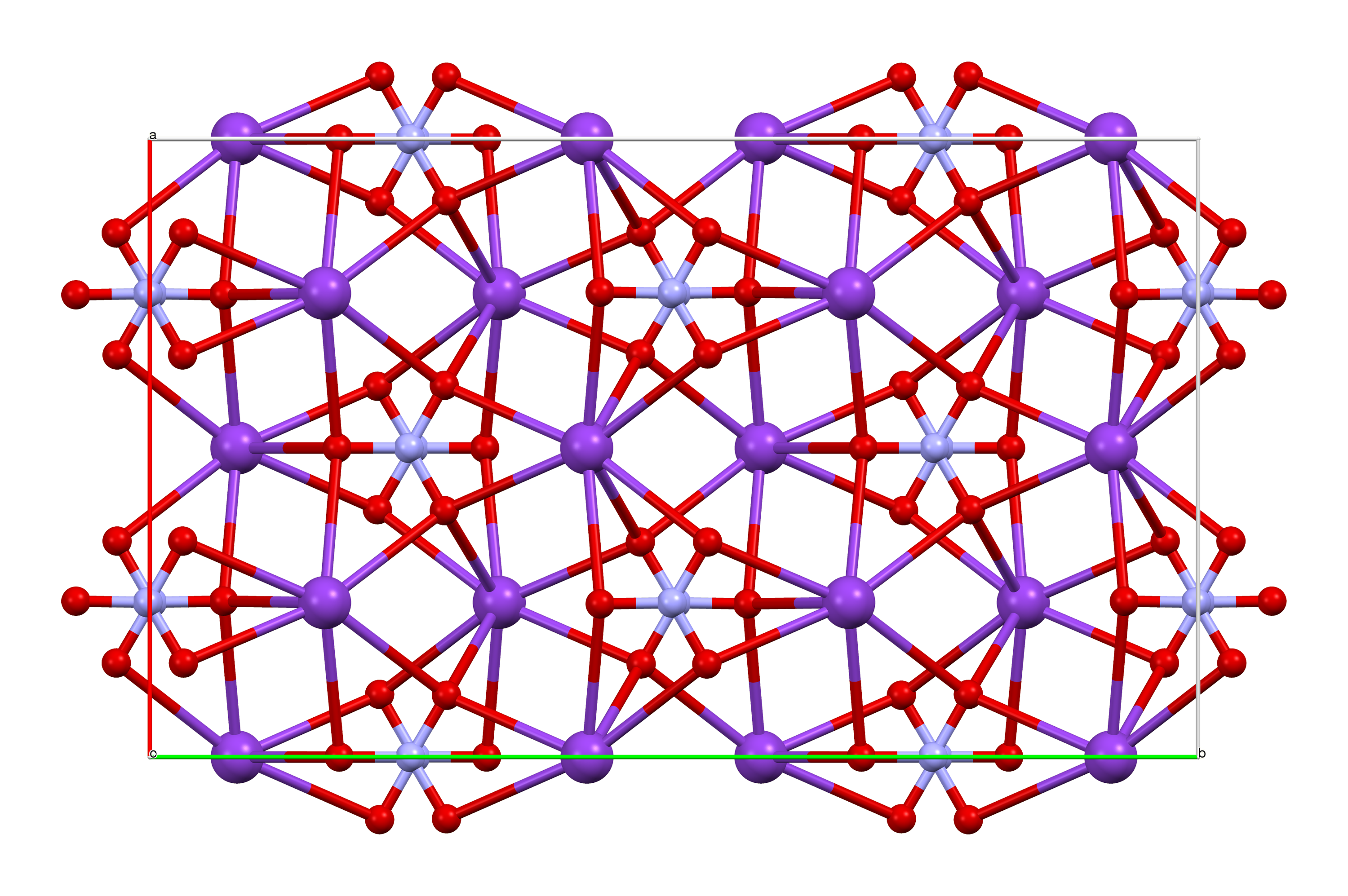
Ammonium Perchlorate
Ammonium perchlorate ("AP") is an inorganic compound with the formula . It is a colorless or white solid that is soluble in water. It is a powerful oxidizer. Combined with a fuel, it can be used as a rocket propellant called ammonium perchlorate composite propellant. Its instability has involved it in accidents such as the PEPCON disaster. Production Ammonium perchlorate (AP) is produced by reaction between ammonia and perchloric acid. This process is the main outlet for the industrial production of perchloric acid. The salt also can be produced by salt metathesis reaction of ammonium salts with sodium perchlorate. This process exploits the relatively low solubility of NH4ClO4, which is about 10% of that for sodium perchlorate.Helmut Vogt, Jan Balej, John E. Bennett, Peter Wintzer, Saeed Akbar Sheikh, Patrizio Gallone "Chlorine Oxides and Chlorine Oxygen Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH. AP crystallises as colorless rhombohedra. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Air–fuel Ratio
Air–fuel ratio (AFR) is the mass ratio of air to a solid, liquid, or gaseous fuel present in a combustion process. The combustion may take place in a controlled manner such as in an internal combustion engine or industrial furnace, or may result in an explosion (e.g., a dust explosion). The air–fuel ratio determines whether a mixture is combustible at all, how much energy is being released, and how much unwanted pollutants are produced in the reaction. Typically a range of air to fuel ratios exists, outside of which ignition will not occur. These are known as the lower and upper explosive limits. In an internal combustion engine or industrial furnace, the air–fuel ratio is an important measure for anti-pollution and performance-tuning reasons. If exactly enough air is provided to completely burn all of the fuel ( stoichiometric combustion), the ratio is known as the stoichiometric mixture, often abbreviated to stoich. Ratios lower than stoichiometric (where the fuel is in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amatol
Amatol is a highly explosive material made from a mixture of TNT and ammonium nitrate. The British name originates from the words ammonium and toluene (the precursor of TNT). Similar mixtures (one part dinitronaphthalene and seven parts ammonium nitrate) were known as Schneiderite in France. Amatol was used extensively during World War I and World War II, typically as an explosive in military weapons such as aircraft bombs, shells, depth charges, and naval mines.Brown, G. I. (1998). ''The Big Bang: A History of Explosives''. Sutton Publishing . pp. 158-163. It was eventually replaced with alternative explosives such as Composition B, Torpex, and Tritonal. Invention Following the Shell Crisis of 1915 in which the UK did not have enough ordnance due to a lack of explosives, a team at the Royal Arsenal laboratories produced a mixture of ammonium nitrate and TNT, known as Amatol for short. Special factories were constructed for the manufacture of ammonium nitrate by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brisance
Brisance (; ) is the shattering capability of a high explosive, determined mainly by its detonation pressure. Application Brisance is of practical importance in explosives engineering for determining the effectiveness of an explosion in blasting and quarrying, and in weaponry such as fragmenting shells, bomb casings, grenades, and plastic explosives. The sand crush test and Trauzl lead block test are commonly used to determine the relative brisance in comparison to TNT (which is considered a standard reference for many purposes). The value of brisance depends on the application. At one extreme, if an explosive is to be used for propulsion, e.g. by driving a piston or a bullet, brisance is likely to be undesirable, because the objective will be to ''move'' the load, not to shatter it or the engine or firearm, nor to produce a loud report. Such propulsive charges are designed to burn controllably, such as carburetted fuel/air mixes in vehicle piston engines, or nitrocellulos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trinitrotriazine
Trinitrotriazine, or 2,4,6-trinitro-1,3,5-triazine, is a theoretical explosive compound. Synthesis of this compound has been elusive despite its simple structure, as conventional nitration of triazine becomes increasingly more difficult as more nitro groups are added. A successful route would more likely proceed by trimerisation of nitryl cyanide. The precursor nitryl cyanide was first synthesized by Rahm et al. in 2014. Trinitrotriazine has a neutral oxygen balance, potentially making it a very powerful explosive, though calculations predict it would be fairly unstable and inferior to the related compound 3,6-dinitro-1,2,4,5-tetrazine.Jinshan Li. An Ab Initio Theoretical Study of 2,4,6-Trinitro-1,3,5-Triazine, 3,6-Dinitro-1,2,4,5-Tetrazine, and 2,5,8-Trinitro-Tri-s-Triazine, more commonly known as RDX. ''Propellants, Explosives, Pyrotechnics.'' December 2008; 33(6):443-447. See also * RDX RDX (Research Department Explosive or Royal Demolition Explosive) or hexogen, among o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Glycol Dinitrate
Ethylene glycol dinitrate, abbreviated EGDN and NGC, also known as Nitroglycol, is a colorless, oily, explosive liquid obtained by nitrating ethylene glycol. It is similar to nitroglycerine in both manufacture and properties, though it is more volatile and less viscous. Unlike nitroglycerine, the chemical has a perfect oxygen balance, meaning that its ideal exothermic decomposition would completely convert it to low energy carbon dioxide Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ..., water, and nitrogen gas, with no excess unreacted substances, without needing to react with anything else. History and production Pure EGDN was first produced by the Belgian chemist Louis Henry (1834–1913) in 1870 by dropping a small amount of ethylene glycol into a mixture of nitric acid, nitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitroglycerine
Nitroglycerin (NG) (alternative spelling nitroglycerine), also known as trinitroglycerol (TNG), nitro, glyceryl trinitrate (GTN), or 1,2,3-trinitroxypropane, is a dense, colorless or pale yellow, oily, explosive liquid most commonly produced by nitrating glycerol with white fuming nitric acid under conditions appropriate to the formation of the nitric acid ester. Chemically, the substance is a nitrate ester rather than a nitro compound, but the traditional name is retained. Discovered in 1846 by Ascanio Sobrero, nitroglycerin has been used as an active ingredient in the manufacture of explosives, namely dynamite, and as such it is employed in the construction, demolition, and mining industries. It is combined with nitrocellulose to form double-based smokeless powder, used as a propellant in artillery and firearms since the 1880s. As is the case for many other explosives, nitroglycerin becomes more and more prone to exploding (i.e. spontaneous decomposition) as the temperature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Perchlorate
Lithium perchlorate is the inorganic compound with the formula LiClO4. This white or colourless crystalline salt is noteworthy for its high solubility in many solvents. It exists both in anhydrous form and as a trihydrate. Applications Inorganic chemistry Lithium perchlorate is used as a source of oxygen in some chemical oxygen generators. It decomposes at about 400 °C, yielding lithium chloride and oxygen: : LiClO4 → LiCl + 2 O2 Over 60% of the mass of the lithium perchlorate is released as oxygen. It has both the highest oxygen to weight and oxygen to volume ratio of all practical perchlorate salts, and higher oxygen to volume ratio than liquid oxygen. Lithium perchlorate is used as an oxidizer in some experimental solid rocket propellants, and to produce red colored flame in pyrotechnic compositions. Organic chemistry LiClO4 is highly soluble in organic solvents, even diethyl ether. Such solutions are employed in Diels–Alder reactions, where it is proposed t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetranitromethane
Tetranitromethane or TNM is an organic oxidizer with chemical formula . Its chemical structure consists of four nitro groups attached to one carbon atom. In 1857 it was first synthesised by the reaction of sodium cyanoacetamide with nitric acid. Uses It has been investigated for use as an oxidizer in bipropellant rockets. Highly purified tetranitromethane cannot be made to explode, but its sensitivity is increased dramatically by oxidizable contaminants, such as anti-freezing additives. The pure substance also has too high a freezing point to remain reliably molten, although the eutectic with dinitrogen tetroxide freezes at the much lower −30 °C and is less explosive than nearly-pure tetranitromethane. Nevertheless, the oxidizer is still too sensitive for any effective use. In the laboratory it is used as a reagent for the detection of double bonds in organic compounds and as a nitrating reagent. It has also found use as an additive to diesel fuel to increas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Nitrate
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula . It is a potassium salt of nitric acid. This salt consists of potassium cations and nitrate anions , and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or ''nitre'' outside the United States). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpetre (or saltpeter in the United States). Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks. It is one of the major constituents of traditional gunpowder (black powder). In processed meats, potassium nitrate reacts with hemoglobin and myoglobin generating a red color. Etymology Nitre, or potassium nitrate, because of its early and global use and production, has many names. As for nitrate, Egyptian and Hebrew words for it had the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chlorate
Sodium chlorate is an inorganic compound with the chemical formula Na ClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride. Several hundred million tons are produced annually, mainly for applications in bleaching pulp to produce high brightness paper. Synthesis Industrially, sodium chlorate is produced by the electrolysis of concentrated sodium chloride solutions. All other processes are obsolete. The sodium chlorate process is not to be confused with the chloralkali process, which is an industrial process for the electrolytic production of sodium hydroxide and chlorine gas. The overall reaction can be simplified to the equation: First, chloride is oxidised to form intermediate hypochlorite, ClO−, which undergoes further oxidation to chlorate along two competing reaction paths: (1) Anodic chlorate formation at the boundary layer between the electrolyte and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |