|
Oxiconazole
Oxiconazole (trade names Oxistat in the US, Oxizole in Canada) is an antifungal medication typically administered in a cream or lotion to treat skin infections, such as athlete's foot, jock itch and ringworm. It can also be prescribed to treat the skin rash known as tinea versicolor, caused by systemic yeast overgrowth (''Candidiasis, Candida'' spp.). It was patented in 1975 and approved for medical use in 1983. Synthesis : Treatment of the ketone (1) with hydroxylamine gives the oxime (2), which is Alkylation#O-alkylation, alkylated with 2,4-dichlorobenzyl chloride (3) in the presence of the strong base sodium hydride to give oxiconazole. See also * Fluconazole References External links MedlinePlus - Oxiconazole Chlorobenzene derivatives Imidazole antifungals Lanosterol 14α-demethylase inhibitors Ketoxime ethers {{genito-urinary-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antifungal Medication
An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as cryptococcal meningitis, and others. Such drugs are usually obtained by a doctor's prescription, but a few are available over the counter (OTC). The evolution of antifungal resistance is a growing threat to health globally. Routes of administration Ocular Indicated when the fungal infection is located in the eye. There is currently only one ocular antifungal available: natamycin. However, various other antifungal agents could be compounded in this formulation. Intrathecal Used occasionally when there's an infection of the central nervous system and other systemic options cannot reach the concentration required in that region for therapeutic benefit. Example(s): amphotericin B. Vaginal This may be used to treat some fungal infec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
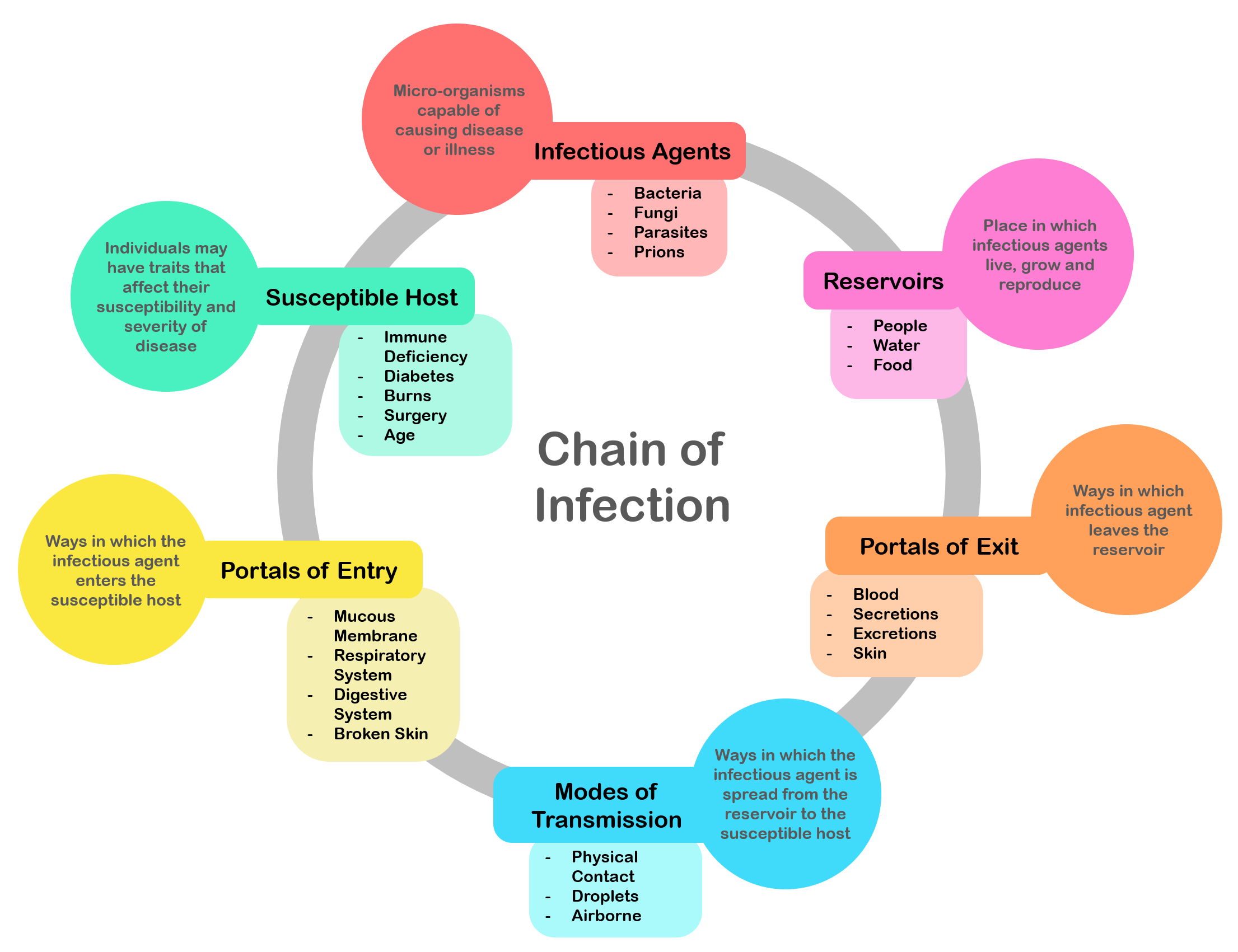
Infection
An infection is the invasion of tissue (biology), tissues by pathogens, their multiplication, and the reaction of host (biology), host tissues to the infectious agent and the toxins they produce. An infectious disease, also known as a transmissible disease or communicable disease, is an Disease#Terminology, illness resulting from an infection. Infections can be caused by a wide range of pathogens, most prominently pathogenic bacteria, bacteria and viruses. Hosts can fight infections using their immune systems. Mammalian hosts react to infections with an Innate immune system, innate response, often involving inflammation, followed by an Adaptive immune system, adaptive response. Treatment for infections depends on the type of pathogen involved. Common medications include: * Antibiotics for bacterial infections. * Antivirals for viral infections. * Antifungals for fungal infections. * Antiprotozoals for protozoan infections. * Antihelminthics for infections caused by parasi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylamine
Hydroxylamine (also known as hydroxyammonia) is an inorganic compound with the chemical formula . The compound exists as hygroscopic colorless crystals.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Professional Publishing Ltd. pp. 431–432. 1997. Hydroxylamine is almost always provided and used as an aqueous solution or more often as one of its salts such as hydroxylammonium sulfate, a water-soluble solid. Hydroxylamine and its salts are consumed almost exclusively to produce Nylon-6. The oxidation of to hydroxylamine is a step in biological nitrification. History Hydroxylamine was first prepared as hydroxylammonium chloride in 1865 by the German chemist Wilhelm Clemens Lossen (1838-1906); he reacted tin and hydrochloric acid in the presence of ethyl nitrate. It was first prepared in pure form in 1891 by the Dutch chemist Lobry de Bruyn and by the French chemist Léon Maurice Crismer (1858-1944). The coordination complex (zin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazole Antifungals
Imidazole (ImH) is an organic compound with the formula . It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole. Many natural products, especially alkaloids, contain the imidazole ring. These imidazoles share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building blocks, such as histidine and the related hormone histamine. Many drugs contain an imidazole ring, such as certain antifungal drugs, the nitroimidazole series of antibiotics, and the sedative midazolam. When fused to a pyrimidine ring, it forms a purine, which is the most widely occurring nitrogen-containing heterocycle in nature. The name "imidazole" was coined in 1887 by the German chemist Arthur Rudolf Hantzsch (1857–1935). Structure and properties Imidazole is a planar 5-membered ring, that exists in two equivalent tautomeric forms because hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorobenzene Derivatives
Chlorobenzene (abbreviated PhCl) is an aryl chloride and the simplest of the chlorobenzenes, consisting of a benzene ring substituted with one chlorine atom. Its chemical formula is C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals. Uses The major use of chlorobenzene is as a precursor for further intermediates such as nitrophenols, nitroanisole, chloroaniline, and phenylenediamines, which are used in the production of herbicides, dyestuffs, chemicals for rubber, and pharmaceuticals. It is also used as a high-boiling solvent in industrial and laboratory applications, for materials such as oils, waxes, resins, and rubber. Chlorobenzene is nitrated on a large scale to give a mixture of 2-nitrochlorobenzene and 4-nitrochlorobenzene, which are separated and used as intermediates in production of other chemicals. These mononitrochlorobenzenes are converted to related 2-nitrophenol, 2-nitroanisole, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluconazole
Fluconazole is an antifungal medication used for a number of fungal infections. These include candidiasis, blastomycosis, coccidioidomycosis, cryptococcosis, histoplasmosis, dermatophytosis, and tinea versicolor. It is also used to prevent candidiasis in those who are at high risk such as following organ transplantation, low birth weight babies, and those with low blood neutrophil counts. It is given either by mouth or by injection into a vein. Common side effects include vomiting, diarrhea, rash, and increased liver enzymes. Serious side effects may include liver problems, QT prolongation, and seizures. During pregnancy it may increase the risk of miscarriage while large doses may cause birth defects. Fluconazole is in the azole antifungal family of medication. It is believed to work by affecting the fungal cellular membrane. Fluconazole was patented in 1981 and came into commercial use in 1988. It is on the World Health Organization's List of Essential M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, silane, germane, ammonia, and methane. It is an ionic material that is insoluble in all solvents (other than molten sodium metal), consistent with the fact that H− ions do not exist in solution. Basic properties and structure NaH is colorless, although samples generally appear grey. NaH is around 40% denser than Na (0.968 g/cm3). NaH, like LiH, KH, RbH, and CsH, adopts the NaCl crystal structure. In this motif, each Na+ ion is surrounded by six H− centers in an octahedral geometry. The ionic radii of H− (146 pm in NaH) and F− (133 pm) are comparable, as judged by the Na−H and Na−F distances. "Inverse sodium hydride" (hydrogen sodide) A very unusual situation occurs in a com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with alkene, olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion (carbanion). The formal "alkyl anion" attacks an electrophile, forming a new covalent bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional group, group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides () with general structure . Oximes are usually generated by the reaction of hydroxylamine with aldehydes () or ketones (). The term ''oxime'' dates back to the 19th century, a combination of the words ''oxygen'' and ''imine''. Structure and properties If the two side-chains on the central carbon are different from each other—either an aldoxime, or a ketoxime with two different "R" groups—the oxime can often have two different geometric stereoisomeric forms according to the E/Z configuration, ''E''/''Z'' configuration. An older terminology of Descriptor (chemistry)#syn, anti, ''syn'' and ''anti'' was used to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' are methyl), with the formula . Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, ''e.g.'', testosterone, and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered retained IUPAC names, although some introdu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Athlete's Foot
Athlete's foot, known medically as ''tinea pedis'', is a common skin infection of the feet caused by a fungus. Signs and symptoms often include itching, scaling, cracking and redness. In rare cases the skin may blister. Athlete's foot fungus may infect any part of the foot, but most often grows between the toes. The next most common area is the bottom of the foot. The same fungus may also affect the nails or the hands. It is a member of the group of diseases known as '' tinea''. Athlete's foot is caused by a number of different funguses, including species of '' Trichophyton'', '' Epidermophyton'', and '' Microsporum''. The condition is typically acquired by coming into contact with infected skin, or fungus in the environment. Common places where the funguses can survive are around swimming pools and in locker rooms. They may also be spread from other animals. Usually diagnosis is made based on signs and symptoms; however, it can be confirmed either by culture or seeing hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |