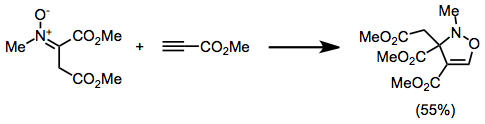|
Oxazolidine-dione
Oxazolidine is a five-membered heterocycle ringwith the formula .The O atom and NH groups are not mutually bonded, in contrast to isoxazolidine. Oxazolidines (emphasis on plural) are derivatives of the parent oxazolidine owing to the presence of substituents on carbon and/or nitrogen. Oxazolines are unsaturated analogues of oxazolidines. Synthesis and reactions First synthesized in the 1800s, oxazolidines are traditionally prepared by condensation of 2-aminoalcohols with aldehydes and ketones. The ready availability of chiral amino alcohols by reduction of amino acids enables the synthesis of chiral oxazolidines. Oxazolidines are prone to hydrolysis, the reverse of their syntheses. Perhaps for this reason, their basicity is rarely discussed. Uses and occurrence Several oxazolidine derivatives occur naturally, Some occur as post translational modifications of proteins. Others are components of alkaloids, a few of which are highly active against some tumors. Examples include te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Famoxadone
Famoxadone is a fungicide to protect agricultural products against various fungal diseases on fruiting vegetables, tomatoes, potatoes, curcurbits, lettuce and grapes.Famoxadone Pesticide Fact Sheet It is used in combination with cymoxanil. Famoxadone is a QI, albeit with a chemistry different from most QIs. (It is an [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diisocyanate
In organic chemistry, isocyanate is the functional group with the formula . Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers. Isocyanates should not be confused with cyanate esters and isocyanides, very different families of compounds. The cyanate (cyanate ester) functional group () is arranged differently from the isocyanate group (). Isocyanides have the connectivity , lacking the oxygen of the cyanate groups. Structure and bonding In terms of bonding, isocyanates are closely related to carbon dioxide (CO2) and carbodiimides (C(NR)2). The C−N=C=O unit that defines isocyanates is planar, and the N=C=O linkage is nearly linear. In phenyl isocyanate, the C=N and C=O distances are respectively 1.195 and 1.173 Å. The C−N=C angle is 134.9° and the N=C=O angle is 173.1� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinclozolin
Vinclozolin (trade names Ronilan, Curalan, Vorlan, Touche) is a common dicarboximide fungicide used to control diseases, such as blights, rots and molds in vineyards, and on fruits and vegetables such as raspberries, lettuce, kiwi, snap beans, and onions. It is also used on turf on golf courses. Two common fungi that vinclozolin is used to protect crops against are ''Botrytis cinerea'' and '' Sclerotinia sclerotiorum''. First registered in 1981, vinclozolin is widely used but its overall application has declined. As a pesticide, vinclozolin is regulated by the United States Environmental Protection Agency (U.S. EPA). In addition to these restrictions within the United States, as of 2006 the use of this pesticide was banned in several countries, including Denmark, Finland, Norway, and Sweden. It has gone through a series of tests and regulations in order to evaluate the risks and hazards to the environment and animals. Among the research, a main finding is that vinclozolin has been ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fungicide
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in losses of yield and quality. Fungicides are used both in agriculture and to fight fungal infections in animals, including humans. Fungicides are also used to control oomycetes, which are not taxonomically/genetically fungi, although sharing similar methods of infecting plants. Fungicides can either be contact, translaminar or systemic. Contact fungicides are not taken up into the plant tissue and protect only the plant where the spray is deposited. Translaminar fungicides redistribute the fungicide from the upper, sprayed leaf surface to the lower, unsprayed surface. Systemic fungicides are taken up and redistributed through the xylem vessels. Few fungicides move to all parts of a plant. Some are locally systemic, and some move upward. Most fungicides that can be bought retail are sold in liquid form, the active ingredient being present at 0.08% i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Group
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ketones and carboxylic acid), as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, such that carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides, chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxama ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazolidinone
The oxazolidones and isoxazolidones are a set of six isomeric five-membered heterocyclic compounds consisting of a carbonyl group, an oxygen atom, a nitrogen atom with a hydrogen atom attached, and two methylene groups. The chemicals differ in the relative position of these groups around the ring, which results in different functional group combinations. 2-Oxazolidinone is the parent compound of several protein synthesis inhibitor antibiotics and the Evans chiral auxiliaries for aldol reactions. Cycloserine Cycloserine, sold under the brand name Seromycin, is a GABA transaminase inhibitor and an antibiotic, used to treat tuberculosis. Specifically it is used, along with other antituberculosis medications, for active drug resistant tuberculosis. ... is an antibiotic based on the 3-isoxazolidone parent. See also * Oxazolidine * Isoxazolidine {{Chemistry index Oxazolidinones Isoxazolidinones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoxazoline
Isoxazoline is a five-membered heterocyclic chemical compound, containing one atom each of oxygen and nitrogen which are located adjacent to one another. The ring was named in-line with the Hantzsch–Widman nomenclature. Isoxazolines are structural isomers of the more common oxazolines and exist in three different isomers depending on the location of the double bond. The relatively weak N-O bond makes isoxazolines prone to ring-opening and rearrangement reactions. Uses There are thousands of compounds containing isoxazoline rings, which are described to have a particular use. A number of naturally occurring isoxazolines with possible anti-cancer activity are produced by marine sponges. Isoxazoline insecticide The isoxazoline class of insecticides was discovered by Nissan. They act by allosterically modulating GABA-gated chloride channels (IRAC group 30) and glutamate-gated chloride channels; this decreases influx of chloride into the post-synaptic neuron, leading to hype ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoxazolidine
Isoxazolidine is the organic compound with the formula (CH2)3(NH)O. It is the parent of a family of compounds called Isoxazolidines, which are saturated C3NO heterocyclic rings where the nitrogen and oxygen occupy adjacent positions (1 and 2). They are the saturated analogues of Isoxazoles, and they are isomeric with oxazolidines, where the N and O are separated by one carbon. Isoxazolidines can be produced by the nitrone-olefin (3+2) cycloaddition reaction. : They represent precursors to 1,3-aminoalcohols. The series Organic Syntheses provides detailed procedures that yield isoxazolidines, e.g., from styrene and N-phenylmaleimide Maleimide is a chemical compound with the formula H2C2(CO)2NH (see diagram). This unsaturated imide is an important building block in organic synthesis. The name is a contraction of maleic acid and imide, the -C(O)NHC(O)- functional group. Malei .... Some isoxazolidines are of medicinal interest.{{Cite journal , doi = 10.1021/acs.chemrev.6b0054 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexamethylene Diisocyanate
Hexamethylene diisocyanate (HDI) is the organic compound with the formula (CH2)6(NCO)2. It is classified as an diisocyanate. It is a colorless liquid. It has sometimes been called HMDI but this not usually done to avoid confusion with Hydrogenated MDI. Synthesis Compared to other commercial diisocyanates, HDI is produced in relatively small quantities, accounting for (with isophorone diisocyanate) only 3.4% of the global diisocyanate market in the year 2000. It is produced by phosgenation of hexamethylene diamine. Applications Aliphatic diisocyanates are used in specialty applications, such as enamel coatings which are resistant to abrasion and degradation by ultraviolet light. These properties are particularly desirable in, for instance, the exterior paint applied to aircraft and vessels. HDI is also sold oligomerized as the trimer or biuret which are used in automotive refinish coatings. Although more viscous in these forms, it reduces the volatility and toxicity. At least 3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Acid Anhydride
An organic acid anhydride is an acid anhydride that is also an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word ''acid'' in the name of the parent carboxylic acid by the word ''anhydride''. Thus, (CH3CO)2O is called ''acetic anhydride.'' ''Mixed'' (or ''unsymmetrical'') acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride"). One or both acyl groups of an acid anhydrid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. Alcohols, with –OH, can act as acids but they are usually very weak. The relative stability of the conjugate base of the acid determines its acidity. Other groups can also confer acidity, usually weakly: the thiol group –SH, the enol group, and the phenol group. In biological systems, organic compounds containing these groups are generally referred to as organic acids. A few common examples include: * Lactic acid * Acetic acid * Formic acid * Citric acid * Oxalic acid * Uric acid * Malic acid * Tartaric acid * Butyric acid * Folic acid Characteristics In general, organic acids are weak acids and do not dissociate completely in water, whereas the strong mineral acids do. Lower molecular mass organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |