|
Organolead Compound
Organolead chemistry is the scientific study of the synthesis and properties of organolead compounds, which are organometallic compounds containing a chemical bond between carbon and lead. The first organolead compound was hexaethyldilead (Pb2(C2H5)6), first synthesized in 1858.''Main Group Metals in Organic Synthesis'' Yamamoto, Hisashi / Oshima, Koichiro (eds.) 2004 Sharing the same group with carbon, lead is tetravalent. Going down the carbon group the C–''X'' (''X'' = C, Si, Ge, Sn, Pb) bond becomes weaker and the bond length larger. The C–Pb bond in tetramethyllead is 222 picometer, pm long with a dissociation energy of 49 calorie, kcal/mole (unit), mol (204 kilojoule, kJ/mol). For comparison the C–Sn bond in tetramethyltin is 214 pm long with dissociation energy 71 kcal/mol (297 kilojoule, kJ/mol). The dominance of Pb(IV) in organolead chemistry is remarkable because inorganic lead compounds tend to have Pb(II) centers. The reason is that with inorganic lead compounds e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum, i.e., under a lower pressure, has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at 100°C (or with scientific precision: ) under standard pressure at sea level, but at at altitude. For a given pressure, different liquids will boiling, boil at different temperatures. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one Atmosphere (unit), atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylmagnesium Chloride
Methylmagnesium chloride is an organometallic compound with the general formula . This highly flammable, colorless, and moisture sensitive compound is the simplest Grignard reagent and is commercially available, usually as a solution in tetrahydrofuran. Synthesis and reactions Relative to the more commonly encountered methylmagnesium bromide and methylmagnesium iodide, methylmagnesium chloride offers the advantages of low equivalent weight and low cost. It is prepared by the reaction of methyl chloride and magnesium in ethyl ether. left, Structure of , which is representative of the species in donor solvents. As with most Grignard reagents, methylmagnesium chloride is highly solvated by ether solvents via coordination from two oxygen atoms to give a tetrahedrally bonded magnesium center. Like methyllithium Methyllithium is the simplest organolithium reagent, with the empirical formula LiCH3. This s-block organometallic compound adopts an oligomeric structure both in s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead Chloride
Lead chloride may refer to: * Lead(II) chloride (plumbous chloride), mineral name: cotunnite Cotunnite is the natural mineral form of lead(II) chloride (PbCl2). Unlike the pure compound, which is white, cotunnite can be white, yellow, or green. The density of mineral samples spans range 5.3–5.8 g/cm3. The hardness on the Mohs scale is 1 .... * Lead(IV) chloride (plumbic chloride) * Hexachloroplumbate(IV) (dianion) {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon–carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. Grignard reagents are rarely isolated as solids. Instead, they are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran using air-free techniques. Grignard reagents are complex with the magnesium atom bonded to two ether ligands as well as the halide and organyl ligands. The discovery of the Grignard reaction in 1900 was recogn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead(II) Chloride
Lead(II) chloride (PbCl2) is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It also occurs naturally in the form of the mineral cotunnite. Structure and properties In solid PbCl2, each lead ion is coordinated by nine chloride ions in a tricapped triangular prism formation — six lie at the vertices of a triangular prism and three lie beyond the centers of each rectangular prism face. The 9 chloride ions are not equidistant from the central lead atom, 7 lie at 280–309 pm and 2 at 370 pm. PbCl2 forms white orthorhombic needles. In the gas phase, PbCl2 molecules have a bent structure with the Cl–Pb–Cl angle being 98° and each Pb–-Cl bond distance being 2.44 Å. Such PbCl2 is emitted from internal combustion engines that use ethylene chloride-tetraethyllead additives for antiknock purposes. PbCl2 is sparingly soluble in water, solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead Tetraacetate
Lead(IV) acetate or lead tetraacetate is an metalorganic compound with chemical formula , often abbreviated as , where Ac is acyl. It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis. Structure In the solid state the lead(IV) centers are coordinated by four acetate ions, which are bidentate, each coordinating via two oxygen atoms. The lead atom is 8 coordinate and the O atoms form a flattened trigonal dodecahedron. Preparation It is typically prepared by treating of red lead with acetic acid and acetic anhydride (), which absorbs water. The net reaction is shown: : The remaining lead(II) acetate can be partially oxidized to the tetraacetate by Cl2, with a by-product: : Reagent in organic chemistry Lead tetraacetate is a strong oxidizing agent, a source of acetyloxy groups, and a general reagent for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Avgas
Avgas (aviation gasoline, also known as aviation spirit in the United Kingdom, UK) is an aviation fuel used in aircraft with spark-ignited internal combustion engines. ''Avgas'' is distinguished from conventional gasoline (petrol) used in motor vehicles, which is termed ''mogas'' (motor gasoline) in an aviation context. Unlike motor gasoline, which has been formulated without lead since the 1970s to allow the use of catalytic converters for pollution reduction, the most commonly used grades of avgas still contain tetraethyllead, tetraethyl lead, a toxic lead-containing additive used to aid in lubrication of the engine, increase octane rating, and prevent engine knocking (spark-knock). There are ongoing efforts to reduce or eliminate the use of lead in aviation gasoline. Kerosene-based jet fuel is formulated to suit the requirements of gas turbine, turbine engines which have no octane requirement and operate over a much wider flight envelope than piston engines. Kerosene is also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
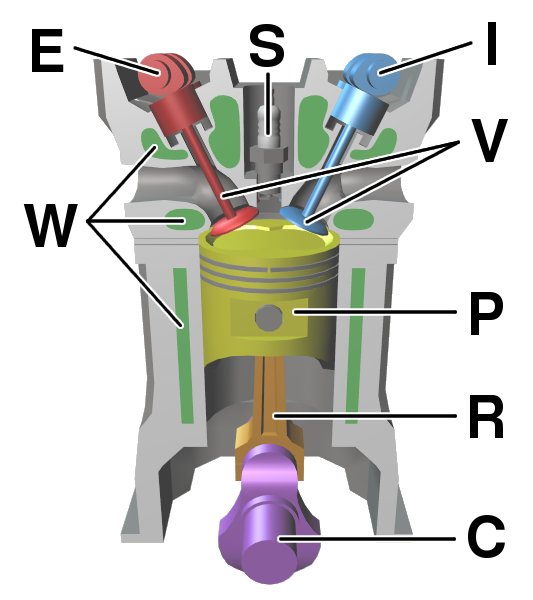
Internal Combustion Engines
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons (reciprocating engine, piston engine), turbine blades (gas turbine), a Wankel engine, rotor (Wankel engine), or a propulsive nozzle, nozzle (jet engine). This force moves the component over a distance. This process transforms chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to. The first commercially successful internal combustion engines were invented in the mid-19th century. The first modern internal combustion engine, the Otto engine, was designed in 1876 by the German engineer Nicolaus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gasoline
Gasoline ( North American English) or petrol ( Commonwealth English) is a petrochemical product characterized as a transparent, yellowish, and flammable liquid normally used as a fuel for spark-ignited internal combustion engines. When formulated as a fuel for engines, gasoline is chemically composed of organic compounds derived from the fractional distillation of petroleum and later chemically enhanced with gasoline additives. It is a high-volume profitable product produced in crude oil refineries. The ability of a particular gasoline blend to resist premature ignition (which causes knocking and reduces efficiency in reciprocating engines) is measured by its octane rating. Tetraethyl lead was once widely used to increase the octane rating but is not used in modern automotive gasoline due to the health hazard. Aviation, off-road motor vehicles, and racing car engines still use leaded gasolines. Other substances are frequently added to gasoline to improve chemical st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiknock Agent
An antiknock agent (also: knock inhibitor) is a gasoline additive used to reduce engine knocking and increase the fuel's octane rating by raising the temperature and pressure at which auto-ignition occurs. The mixture known as gasoline or petrol, when used in high compression internal combustion engines, has a tendency to knock (also called "pinging" or "pinking") and/or to ignite early before the correctly timed spark occurs (''pre-ignition'', refer to engine knocking). Notable early antiknock agents, especially tetraethyllead, added to gasoline included large amounts of toxic lead. The chemical was responsible for global negative impacts on health, and the phase out of leaded gasoline from the 1970s onward was reported by the United Nations Environmental Programme to be responsible for "$2.4 trillion in annual benefits, 1.2 million fewer premature deaths, higher overall intelligence and 58 million fewer crimes." Some other chemicals used as gasoline additives are thought to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |