|
Organic Geochemistry
Organic geochemistry is the study of the impacts and processes that organisms have had on the Earth. It is mainly concerned with the composition and mode of origin of organic matter in rocks and in bodies of water. The study of organic geochemistry is traced to the work of Alfred E. Treibs, "the father of organic geochemistry." Treibs first isolated metalloporphyrins from petroleum. This discovery established the biological origin of petroleum, which was previously poorly understood. Metalloporphyrins in general are highly stable organic compounds, and the detailed structures of the extracted derivatives made clear that they originated from chlorophyll. Applications Energy Petroleum The relationship between the occurrence of organic compounds in sedimentary deposits and petroleum deposits has long been of interest. Studies of ancient sediments and rock provide insights into the origins and sources of oil and petroleum, as well as the biochemical antecedents of life. Oil spills in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alfred E
Alfred may refer to: Arts and entertainment *''Alfred J. Kwak'', Dutch-German-Japanese anime television series *Alfred (Arne opera), ''Alfred'' (Arne opera), a 1740 masque by Thomas Arne *Alfred (Dvořák), ''Alfred'' (Dvořák), an 1870 opera by Antonín Dvořák *"Alfred (Interlude)" and "Alfred (Outro)", songs by Eminem from the 2020 album ''Music to Be Murdered By'' Business and organisations * Alfred, a radio station in Shaftesbury, England *Alfred Music, an American music publisher *Alfred University, New York, U.S. *The Alfred Hospital, a hospital in Melbourne, Australia People * Alfred (name) includes a list of people and fictional characters called Alfred * Alfred the Great (848/49 – 899), or Alfred I, a king of the West Saxons and of the Anglo-Saxons Places Antarctica * Mount Alfred (Antarctica) Australia * Alfredtown, New South Wales * County of Alfred, South Australia Canada * Alfred and Plantagenet, Ontario ** Alfred, Ontario, a community in Alfred and Plantag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology The word ''pyrolysis'' is coined from the Greek language, Greek-derived morpheme, elements ''pyro-'' (from Ancient Greek : - "fire, heat, fever") and ''lysis'' ( : - "separation, loosening"). Applications Pyrolysis is most commonly used in the treatment of organic compound, organic materials. It is one of the processes involved in the charring of wood or pyrolysis of biomass. In general, pyrolysis of organic substances produces volatile products and leaves Char (chemistry), char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly carbon as the residue, is called carbonization. Pyrolysis is considered one of the steps in the processes of gasification or combustion. Laypeople often confuse pyrolysis gas with syngas. Pyrolysis gas has a high percentage of heavy tar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Climate Forcing
Radiative forcing (or climate forcing) is a concept used to quantify a change to the Earth's energy budget, balance of energy flowing through a planetary atmosphere. Various factors contribute to this change in energy balance, such as concentrations of Greenhouse gas, greenhouse gases and Aerosol, aerosols, and changes in albedo, surface albedo and solar irradiance. In more technical terms, it is defined as "the change in the net, downward minus upward, radiative flux (expressed in W/m2) due to a change in an external driver of climate change." These external drivers are distinguished from climate change feedback, feedbacks and Climate variability and change, variability that are internal to the climate system, and that further influence the direction and magnitude of Earth's energy imbalance, imbalance. Radiative forcing on Earth is meaningfully evaluated at the tropopause and at the top of the stratosphere. It is quantified in units of watts per square meter, and often summarized a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aerosol Movement
An aerosol is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be generated from natural or human causes. The term ''aerosol'' commonly refers to the mixture of particulates in air, and not to the particulate matter alone. Examples of natural aerosols are fog, mist or dust. Examples of human caused aerosols include particulate air pollutants, mist from the discharge at hydroelectric dams, irrigation mist, perfume from atomizers, smoke, dust, sprayed pesticides, and medical treatments for respiratory illnesses. Several types of atmospheric aerosol have a significant effect on Earth's climate: volcanic, desert dust, sea-salt, that originating from biogenic sources and human-made. Volcanic aerosol forms in the stratosphere after an eruption as droplets of sulfuric acid that can prevail for up to two years, and reflect sunlight, lowering temperature. Desert dust, mineral particles blown to high altitudes, absorb heat and may be responsib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to naturally occurring petroleum, natural gas and coal, or their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are either carbon dioxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Late Cretaceous Period
The Late Cretaceous (100.5–66 Ma) is the more recent of two epochs into which the Cretaceous Period is divided in the geologic time scale. Rock strata from this epoch form the Upper Cretaceous Series. The Cretaceous is named after ''creta'', the Latin word for the white limestone known as chalk. The chalk of northern France and the white cliffs of south-eastern England date from the Cretaceous Period. Climate During the Late Cretaceous, the climate was warmer than present, although throughout the period a cooling trend is evident. The tropics became restricted to equatorial regions and northern latitudes experienced markedly more seasonal climatic conditions. Geography Due to plate tectonics, the Americas were gradually moving westward, causing the Atlantic Ocean to expand. The Western Interior Seaway divided North America into eastern and western halves; Appalachia and Laramidia. India maintained a northward course towards Asia. In the Southern Hemisphere, Australia and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Isotopes
Uranium (U) is a naturally occurring radioactive element (radioelement) with no stable isotopes. It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in Earth's crust. The decay product uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors. In addition to isotopes found in nature or nuclear reactors, many isotopes with far shorter half-lives have been produced, ranging from U to U (except for U). The standard atomic weight of natural uranium is . Natural uranium consists of three main isotopes, U (99.2739–99.2752% natural abundance), U (0.7198–0.7202%), and U (0.0050–0.0059%). All three isotopes are radioactive (i.e., they are radioisotopes), and the most abundant and stable is uranium-238, with a half-life of (about the age of the Earth). Uranium-238 is an alpha emitter, decaying through the 18-member uranium series into lead-206. The decay series of uran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
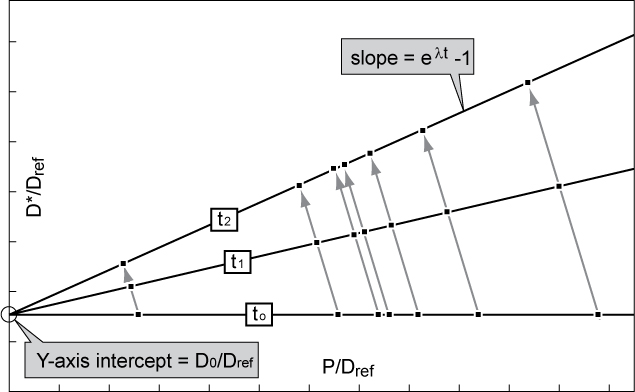
Isochron Dating
Isochron dating is a common technique of radiometric dating and is applied to date certain events, such as crystallization, metamorphism, shock events, and differentiation of precursor melts, in the history of rocks. Isochron dating can be further separated into ''mineral isochron dating'' and ''whole rock isochron dating''; both techniques are applied frequently to date terrestrial and extraterrestrial rocks (meteorites and Moon rocks). The advantage of isochron dating as compared to simple radiometric dating techniques is that no assumptions are needed about the initial amount of the daughter nuclide in the radioactive decay sequence. Indeed, the initial amount of the daughter product can be determined using isochron dating. This technique can be applied if the daughter element has at least one stable isotope other than the daughter isotope into which the parent nuclide decays. Basis for method All forms of isochron dating assume that the source of the rock or rocks contained ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isochron
In the mathematical theory of dynamical systems, an isochron is a set of initial conditions for the system that all lead to the same long-term behaviour. Mathematical isochron An introductory example Consider the ordinary differential equation for a solution y(t) evolving in time: : \frac + \frac = 1 This ordinary differential equation (ODE) needs two initial conditions at, say, time t=0. Denote the initial conditions by y(0)=y_0 and dy/dt(0)=y'_0 where y_0 and y'_0 are some parameters. The following argument shows that the isochrons for this system are here the straight lines y_0+y'_0=\mbox. The general solution of the above ODE is :y=t+A+B\exp(-t) Now, as time increases, t\to\infty, the exponential terms decays very quickly to zero (exponential decay). Thus ''all'' solutions of the ODE quickly approach y\to t+A. That is, ''all'' solutions with the same A have the same long term evolution. The exponential decay of the B\exp(-t) term brings together a host of soluti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Chromatography-mass Spectrometry
Gas is a state of matter that has neither a fixed volume nor a fixed shape and is a compressible fluid. A ''pure gas'' is made up of individual atoms (e.g. a noble gas like neon) or molecules of either a single type of atom ( elements such as oxygen) or from different atoms ( compounds such as carbon dioxide). A ''gas mixture'', such as air, contains a variety of pure gases. What distinguishes gases from liquids and solids is the vast separation of the individual gas particles. This separation can make some gases invisible to the human observer. The gaseous state of matter occurs between the liquid and plasma states, the latter of which provides the upper-temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention. High-density atomic gases super-cooled to very low temperatures are classified by their statistical behavior as either Bose gases or Fermi gases. For a comprehensive listing of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Cycle-cute Diagram
Carbon () is a chemical element; it has symbol C and atomic number 6. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes occur naturally, C and C being stable, while C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the few elements known since antiquity. Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon NMR
Carbon-13 (C13) nuclear magnetic resonance (most commonly known as carbon-13 NMR spectroscopy or 13C NMR spectroscopy or sometimes simply referred to as carbon NMR) is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It is analogous to proton NMR ( NMR) and allows the identification of carbon atoms in an organic molecule just as proton NMR identifies hydrogen atoms. 13C NMR detects only the isotope. The main carbon isotope, does not produce an NMR signal. Although ca. 1 mln. times less sensitive than 1H NMR spectroscopy, 13C NMR spectroscopy is widely used for characterizing organic and organometallic compounds, primarily because 1H-decoupled 13C-NMR spectra are more simple, have a greater sensitivity to differences in the chemical structure, and, thus, are better suited for identifying molecules in complex mixtures. At the same time, such spectra lack quantitative information about the atomic ratios of different types of carbon nuclei, because nuclea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |