|
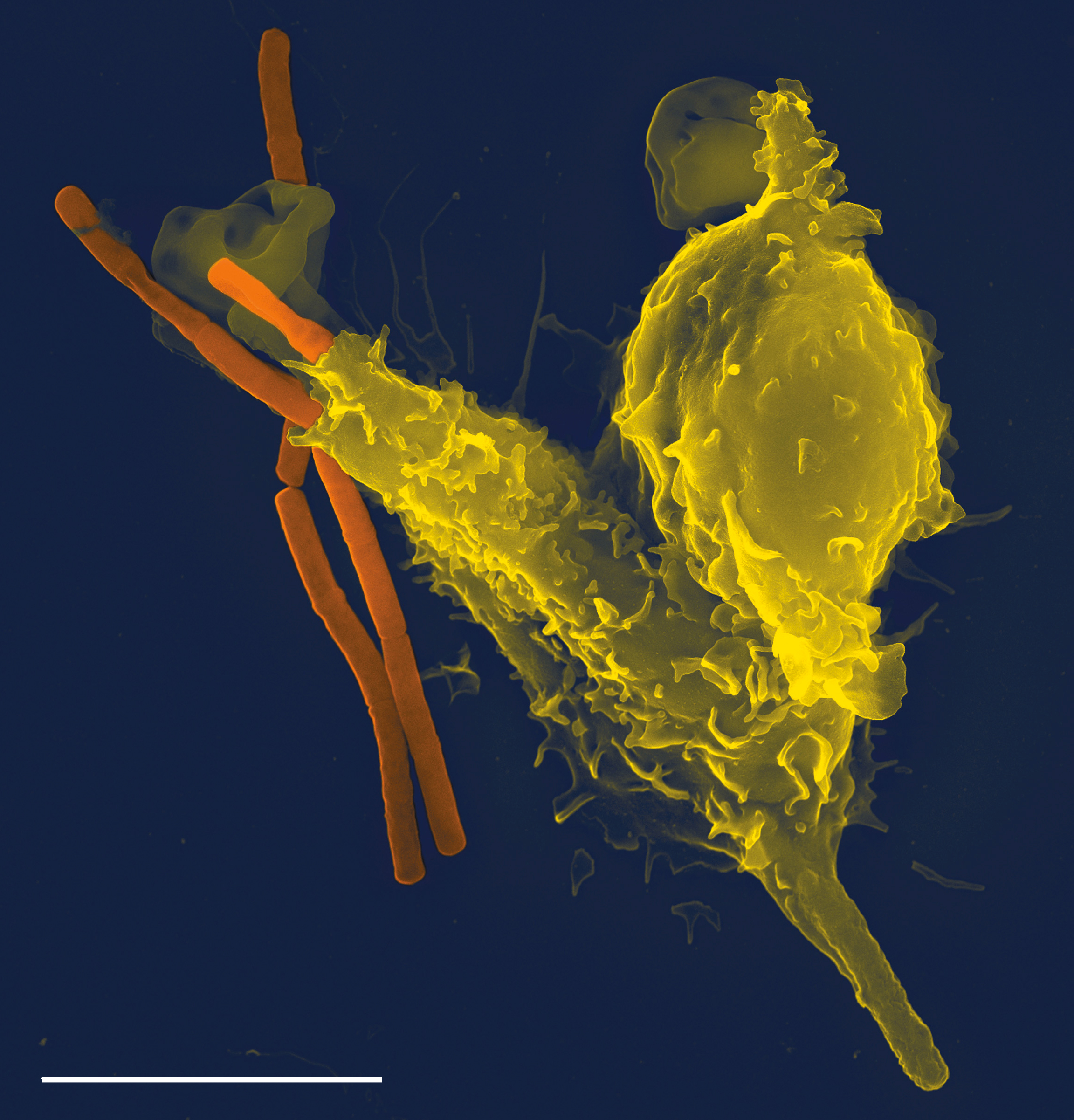
Opsonize
Opsonins are extracellular proteins that, when bound to substances or cells, induce phagocytes to phagocytose the substances or cells with the opsonins bound. Thus, opsonins act as tags to label things in the body that should be phagocytosed (i.e. eaten) by phagocytes (cells that specialise in phagocytosis, i.e. cellular eating). Different types of things ("targets") can be tagged by opsonins for phagocytosis, including: pathogens (such as bacteria), cancer cells, aged cells, dead or dying cells (such as apoptotic cells), excess synapses, or protein aggregates (such as amyloid plaques). Opsonins help clear pathogens, as well as dead, dying and diseased cells. Opsonins were discovered and named "opsonins" in 1904 by Wright and Douglas, who found that incubating bacteria with blood plasma enabled phagocytes to phagocytose (and thereby destroy) the bacteria. They concluded that: “We have here conclusive proof that the blood fluids modify the bacteria in a manner which renders them ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Macrophage-1 Antigen
Macrophage-1 antigen (or integrin αMβ2 or macrophage integrin or Mac-1) is a complement receptor ("CR3") consisting of CD11b (integrin αM) and CD18 (integrin β2). The integrin α chain is noncovalently bound to the integrin β chain. It binds to iC3b and can be involved in cellular adhesion, binding to the intercellular adhesion molecule-1 (ICAM-1). CR3 causes phagocytosis and destruction of cells opsonized with iC3b. CR3 and CR4 are thought to exhibit overlapping functions; however, the distinct binding sites to iC3b suggests differences in their functions. Additionally, CR3 has been shown to have therapeutic promise. Function Macrophage-1 antigen (hereafter complement receptor 3 or CR3) (CD11b/CD18) is a human cell surface receptor found on B and T lymphocytes, polymorphonuclear leukocytes (mostly neutrophils), NK cells, and mononuclear phagocytes like macrophages. CR3 is a pattern recognition receptor, capable of recognizing and binding to many molecules found on the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Fc Receptor
In immunology, an Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc (fragment crystallizable) region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection. Classes There are several different types of Fc receptors (abbreviated FcR), which are classified based on th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Complement Receptor 2
Complement receptor type 2 (CR2), also known as complement C3d receptor, Epstein–Barr virus receptor, and CD21 (cluster of differentiation 21), is a protein that in humans is encoded by the CR2 gene. CR2 is involved in the complement system. It binds to iC3b (inactive derivative of C3b), C3dg, or C3d.Frank K, Atkinson JP (2001). "Complement system." In Austen KF, Frank K, Atkinson JP, Cantor H. eds. ''Samter's Immunologic Diseases, 6th ed. Vol. 1,'' p. 281–298, Philadelphia: Lippincott Williams & Wilkins, . B cells express CR2 on their surfaces, allowing the complement system to play a role in B-cell activation and maturation. Interactions Complement receptor 2 interacts with CD19, and, on mature B cells, forms a complex with CD81 (TAPA-1). The CR2-CD19-CD81 complex is often called the B cell co-receptor complex,Abbas AK, Lichtman AH (2003). ''Cellular and Molecular Immunology, 5th ed.'' Philadelphia: Saunders, because CR2 binds to opsonized antigens through attached C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Complement Receptor 1
Complement receptor type 1 (CR1) also known as C3b/C4b receptor or CD35 (cluster of differentiation 35) is a protein that in humans is encoded by the ''CR1'' gene. This gene is a member of the regulators of complement activation (RCA) family and is located in the 'cluster RCA' region of chromosome 1. The gene encodes a monomeric single-pass type I membrane glycoprotein found on erythrocytes, leukocytes, glomerular podocytes, hyalocytes, and splenic follicular dendritic cells. The Knops blood group system is a system of antigens located on this protein. The protein mediates cellular binding to particles and immune complexes that have activated complement. Decreases in expression of this protein and/or mutations in its gene have been associated with gallbladder carcinomas, mesangiocapillary glomerulonephritis, systemic lupus erythematosus and sarcoidosis. Mutations in this gene have also been associated with a reduction in ''Plasmodium falciparum'' rosetting, conferring protect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Natural Killer Cell
Natural killer cells, also known as NK cells, are a type of cytotoxic lymphocyte critical to the innate immune system. They are a kind of large granular lymphocytes (LGL), and belong to the rapidly expanding family of known innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans. The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cells, stressed cells, tumor cells, and other intracellular pathogens based on signals from several activating and inhibitory receptors. Most immune cells detect the antigen presented on MHC class I, major histocompatibility complex I (MHC-I) on infected cell surfaces, but NK cells can recognize and kill stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Immune System
The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to bacteria, as well as Tumor immunology, cancer cells, Parasitic worm, parasitic worms, and also objects such as wood splinters, distinguishing them from the organism's own healthy biological tissue, tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use humoral immunity, molecules and cell-mediated immunity, cells to perform their functions. Nearly all organisms have some kind of immune system. Bacteria have a rudimentary immune system in the form of enzymes that protect against bacteriophage, viral infections. Other basic immune mechanisms evolved in ancien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Fragment Crystallizable Region
The fragment crystallizable region (Fc region) is the tail region of an antibody that interacts with cell surface receptors called Fc receptors and some proteins of the complement system. This region allows antibodies to activate the immune system, for example, through binding to Fc receptors. In IgG, IgA and IgD antibody isotypes, the Fc region is composed of two identical protein fragments, derived from the second and third constant domains of the antibody's two heavy chains; IgM and IgE Fc regions contain three heavy chain constant domains (CH domains 2–4) in each polypeptide chain. The Fc regions of IgGs bear a highly conserved N-glycosylation site. Glycosylation of the Fc fragment is essential for Fc receptor-mediated activity. The N-glycans attached to this site are predominantly core- fucosylated diantennary structures of the complex type. In addition, small amounts of these N-glycans also bear bisecting GlcNAc and α-2,6 linked sialic acid residues. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Fragment Antigen-binding
The fragment antigen-binding region (Fab region) is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. The variable domain contains the paratope (the antigen-binding site), comprising a set of complementarity-determining regions, at the amino terminal end of the monomer. Each arm of the Y thus binds an epitope on the antigen. Preparation In an experimental setting, Fc and Fab fragments can be generated in the laboratory. The enzyme papain can be used to cleave an immunoglobulin monomer into two Fab fragments and an Fc fragment. Conversely, the enzyme pepsin cleaves below the hinge region, so the result instead is a F(ab')2 fragment and a pFc' fragment. Recently another enzyme for generation of F(ab')2 has been commercially available. The enzyme IdeS (Immunoglobulin degrading enzyme from ''Streptococcus pyogenes'', trade name FabRICATOR) cleaves IgG in a sequence specific manner a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Pathogen-associated Molecular Pattern
Pathogen-associated molecular patterns (PAMPs) are small molecular motifs conserved within a class of microbes, but not present in the host. They are recognized by toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) in both plants and animals. This allows the innate immune system to recognize pathogens and thus, protect the host from infection. This initiation of the immune response consists of the secretion of inflammatory cytokines and chemokines. PAMPs can initiate the maturation of immune cells, which can travel to the primary lymph node and trigger the adaptive immune system that involves the production of antibodies against specific antigens. Although the term "PAMP" is relatively new, the concept that molecules derived from microbes must be detected by receptors from multicellular organisms has been held for many decades, and references to an "endotoxin receptor" are found in much of the older literature. The recognition of PAMPs by the PRRs triggers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Complement Component 4
Complement component 4 (C4), in humans, is a protein involved in the intricate complement system, originating from the human leukocyte antigen (HLA) system. It serves a number of critical functions in immunity, tolerance, and autoimmunity with the other numerous components. Furthermore, it is a crucial factor in connecting the recognition pathways of the overall system instigated by antibody-antigen (Ab-Ag) complexes to the other effector proteins of the innate immune response. For example, the severity of a dysfunctional complement system can lead to fatal diseases and infections. Complex variations of it can also lead to schizophrenia. The C4 protein was thought to derive from a simple two-locus allelic model, which however has been replaced by a much more sophisticated multimodular RCCX gene complex model which contain long and short forms of the C4A or C4B genes usually in tandem RCCX cassettes with copy number variation, that somewhat parallels variation in the levels of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
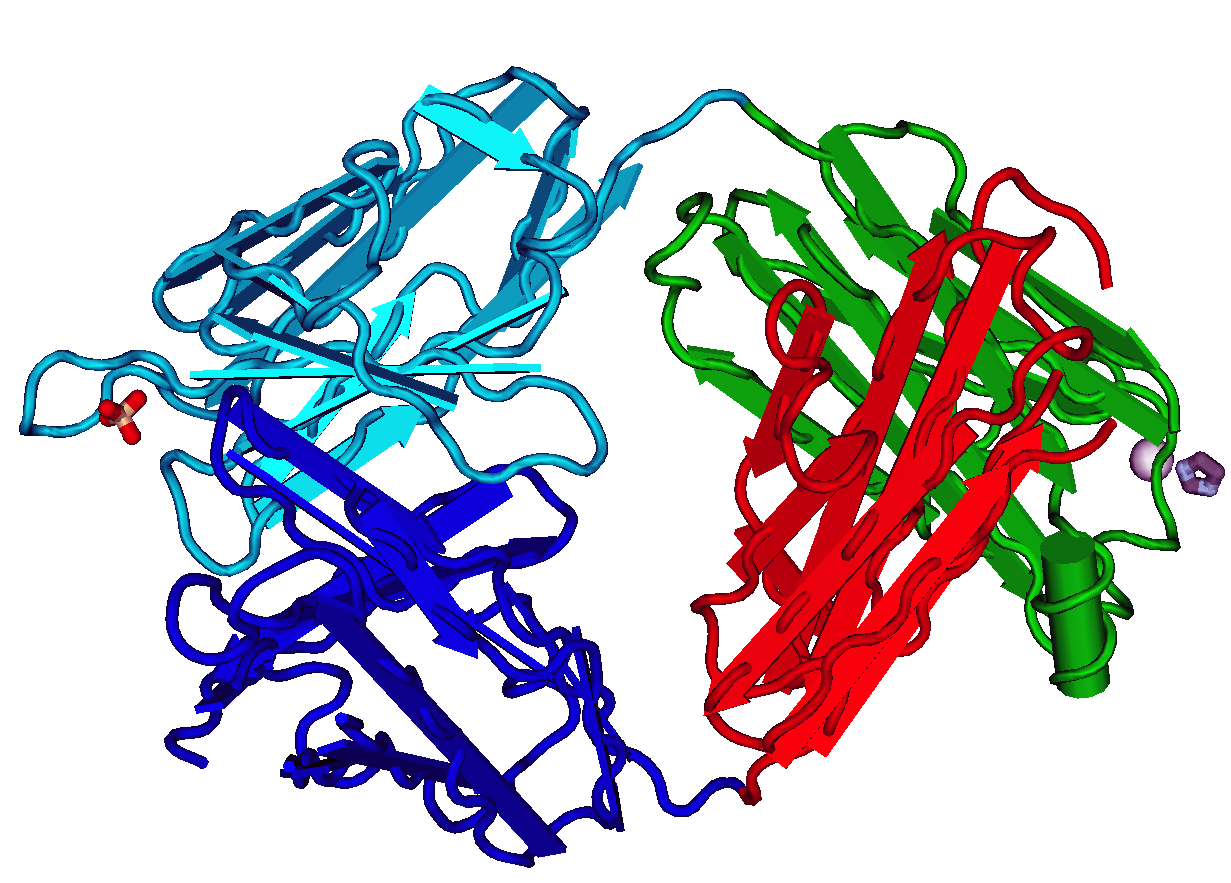
Complement Component 1q
The complement component 1q (or simply C1q) is a protein complex involved in the complement system, which is part of the innate immune system. C1q together with C1r and C1s form the C1 complex. Antibodies of the adaptive immune system can bind antigen, forming an antigen-antibody complex. When C1q binds antigen-antibody complexes, the C1 complex becomes activated. Activation of the C1 complex initiates the classical complement pathway of the complement system. The antibodies IgM and all IgG subclasses except IgG4 are able to initiate the complement system. Structure C1q is a 460 kDa protein formed from 18 peptide chains in 3 subunits of 6. Each 6 peptide subunit consists of a Y-shaped pair of triple peptide helices joined at the stem and ending in a globular non-helical head. The 80-amino acid helical component of each triple peptide contain many Gly-X-Y sequences, where X and Y are proline, isoleucine, or hydroxylysine; they, therefore, strongly resemble collagen fibr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Immunoglobulin M
Immunoglobulin M (IgM) is the largest of several isotypes of antibodies (also known as immunoglobulin) that are produced by vertebrates. IgM is the first antibody to appear in the response to initial exposure to an antigen; causing it to also be called an acute phase antibody. In humans and other mammals that have been studied, plasmablasts in the spleen are the main source of specific IgM production. History In 1937, an antibody was observed in horses hyper-immunized with pneumococcus polysaccharide that was much larger in size than the typical rabbit γ-globulin, with a molecular weight of 990,000 daltons. In accordance with its larger size, the new antibody was originally referred to as γ-macroglobulin, and subsequently termed IgM—M for “macro”. The V domains of normal immunoglobulin are highly heterogeneous, reflecting their role in protecting against the great variety of infectious microbes, and this heterogeneity impeded detailed structural analysis of IgM. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |