|
Opsonization
Opsonins are extracellular proteins that, when bound to substances or cells, induce phagocytes to phagocytose the substances or cells with the opsonins bound. Thus, opsonins act as tags to label things in the body that should be phagocytosed (i.e. eaten) by phagocytes (cells that specialise in phagocytosis, i.e. cellular eating). Different types of things ("targets") can be tagged by opsonins for phagocytosis, including: pathogens (such as bacteria), cancer cells, aged cells, dead or dying cells (such as apoptotic cells), excess synapses, or protein aggregates (such as amyloid plaques). Opsonins help clear pathogens, as well as dead, dying and diseased cells. Opsonins were discovered and named "opsonins" in 1904 by Wright and Douglas, who found that incubating bacteria with blood plasma enabled Phagocyte, phagocytes to phagocytose (and thereby destroy) the bacteria. They concluded that: “We have here conclusive proof that the blood fluids modify the bacteria in a manner which re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as pathogenic bacteria, bacteria and viruses, including those that cause disease. Each individual antibody recognizes one or more specific antigens, and antigens of virtually any size and chemical composition can be recognized. Antigen literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each of the branching chains comprising the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" the antigen (or a microbe or an infected cell bearing such an antigen) for attack by cells of the immune system, or can neutralize it directly (for example, by blocking a p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adaptive Immune System
The adaptive immune system (AIS), also known as the acquired immune system, or specific immune system is a subsystem of the immune system that is composed of specialized cells, organs, and processes that eliminate pathogens specifically. The acquired immune system is one of the two main immunity strategies found in vertebrates (the other being the innate immune system). Like the innate system, the adaptive immune system includes both humoral immunity components and cell-mediated immunity components and destroys invading pathogens. Unlike the innate immune system, which is pre-programmed to react to common broad categories of pathogen, the adaptive immune system is highly specific to each particular pathogen the body has encountered. Adaptive immunity creates immunological memory after an initial response to a specific pathogen, and leads to an enhanced response to future encounters with that pathogen. Antibodies are a critical part of the adaptive immune system. Adaptive im ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
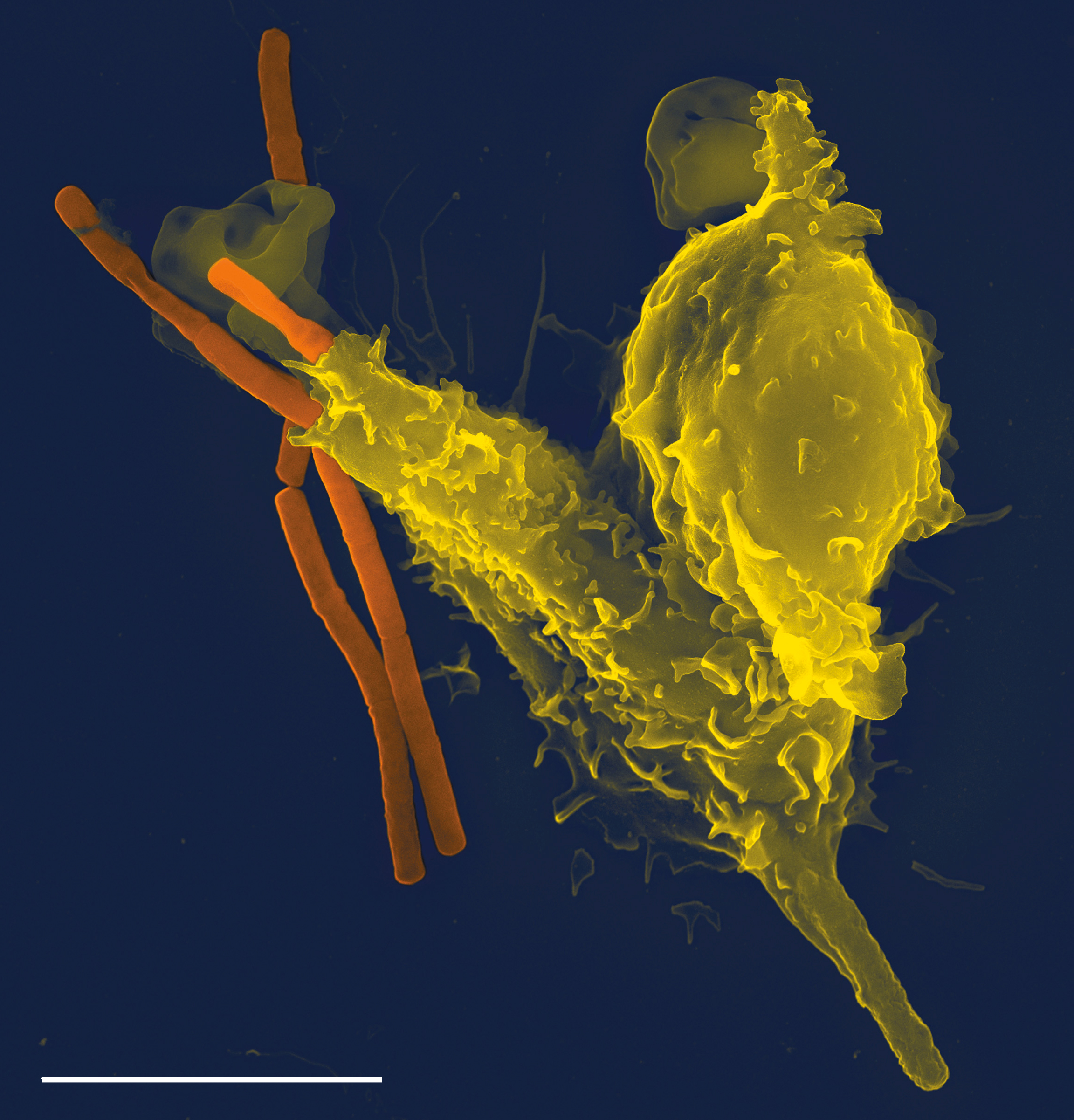
Phagocytes
Phagocytes are cell (biology), cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or Apoptosis, dying cells. Their name comes from the Greek language, Greek ', "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek ''kutos,'' "hollow vessel". They are essential for fighting infections and for subsequent Immunity (medical), immunity. Phagocytes are important throughout the animal kingdom and are highly developed within vertebrates. One litre of human blood contains about six billion phagocytes. They were discovered in 1882 by Élie Metchnikoff, Ilya Ilyich Mechnikov while he was studying starfish larvae. To reach the site of infection, phagocytes leave the bloodstream and enter the affected tissues. Signals from the infection cause the endothelial cells that line the blood vessels to make a protein called selectin, which neutrophils stick to on passing by. Other signals called vasodilators loosen the junctions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fragment Crystallizable Region
The fragment crystallizable region (Fc region) is the tail region of an antibody that interacts with cell surface receptors called Fc receptors and some proteins of the complement system. This region allows antibodies to activate the immune system, for example, through binding to Fc receptors. In IgG, IgA and IgD antibody isotypes, the Fc region is composed of two identical protein fragments, derived from the second and third constant domains of the antibody's two heavy chains; IgM and IgE Fc regions contain three heavy chain constant domains (CH domains 2–4) in each polypeptide chain. The Fc regions of IgGs bear a highly conserved N-glycosylation site. Glycosylation of the Fc fragment is essential for Fc receptor-mediated activity. The N-glycans attached to this site are predominantly core- fucosylated diantennary structures of the complex type. In addition, small amounts of these N-glycans also bear bisecting GlcNAc and α-2,6 linked sialic acid residues. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pathogen-associated Molecular Pattern
Pathogen-associated molecular patterns (PAMPs) are small molecular motifs conserved within a class of microbes, but not present in the host. They are recognized by toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) in both plants and animals. This allows the innate immune system to recognize pathogens and thus, protect the host from infection. This initiation of the immune response consists of the secretion of inflammatory cytokines and chemokines. PAMPs can initiate the maturation of immune cells, which can travel to the primary lymph node and trigger the adaptive immune system that involves the production of antibodies against specific antigens. Although the term "PAMP" is relatively new, the concept that molecules derived from microbes must be detected by receptors from multicellular organisms has been held for many decades, and references to an "endotoxin receptor" are found in much of the older literature. The recognition of PAMPs by the PRRs triggers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrophage-1 Antigen
Macrophage-1 antigen (or integrin αMβ2 or macrophage integrin or Mac-1) is a complement receptor ("CR3") consisting of CD11b (integrin αM) and CD18 (integrin β2). The integrin α chain is noncovalently bound to the integrin β chain. It binds to iC3b and can be involved in cellular adhesion, binding to the intercellular adhesion molecule-1 (ICAM-1). CR3 causes phagocytosis and destruction of cells opsonized with iC3b. CR3 and CR4 are thought to exhibit overlapping functions; however, the distinct binding sites to iC3b suggests differences in their functions. Additionally, CR3 has been shown to have therapeutic promise. Function Macrophage-1 antigen (hereafter complement receptor 3 or CR3) (CD11b/CD18) is a human cell surface receptor found on B and T lymphocytes, polymorphonuclear leukocytes (mostly neutrophils), NK cells, and mononuclear phagocytes like macrophages. CR3 is a pattern recognition receptor, capable of recognizing and binding to many molecules found on the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complement Receptor 1
Complement receptor type 1 (CR1) also known as C3b/C4b receptor or CD35 (cluster of differentiation 35) is a protein that in humans is encoded by the ''CR1'' gene. This gene is a member of the regulators of complement activation (RCA) family and is located in the 'cluster RCA' region of chromosome 1. The gene encodes a monomeric single-pass type I membrane glycoprotein found on erythrocytes, leukocytes, glomerular podocytes, hyalocytes, and splenic follicular dendritic cells. The Knops blood group system is a system of antigens located on this protein. The protein mediates cellular binding to particles and immune complexes that have activated complement. Decreases in expression of this protein and/or mutations in its gene have been associated with gallbladder carcinomas, mesangiocapillary glomerulonephritis, systemic lupus erythematosus and sarcoidosis. Mutations in this gene have also been associated with a reduction in ''Plasmodium falciparum'' rosetting, conferring protect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complement Component 4
Complement component 4 (C4), in humans, is a protein involved in the intricate complement system, originating from the human leukocyte antigen (HLA) system. It serves a number of critical functions in immunity, tolerance, and autoimmunity with the other numerous components. Furthermore, it is a crucial factor in connecting the recognition pathways of the overall system instigated by antibody-antigen (Ab-Ag) complexes to the other effector proteins of the innate immune response. For example, the severity of a dysfunctional complement system can lead to fatal diseases and infections. Complex variations of it can also lead to schizophrenia. The C4 protein was thought to derive from a simple two-locus allelic model, which however has been replaced by a much more sophisticated multimodular RCCX gene complex model which contain long and short forms of the C4A or C4B genes usually in tandem RCCX cassettes with copy number variation, that somewhat parallels variation in the levels of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complement Component 1q
The complement component 1q (or simply C1q) is a protein complex involved in the complement system, which is part of the innate immune system. C1q together with C1r and C1s form the C1 complex. Antibodies of the adaptive immune system can bind antigen, forming an antigen-antibody complex. When C1q binds antigen-antibody complexes, the C1 complex becomes activated. Activation of the C1 complex initiates the classical complement pathway of the complement system. The antibodies IgM and all IgG subclasses except IgG4 are able to initiate the complement system. Structure C1q is a 460 kDa protein formed from 18 peptide chains in 3 subunits of 6. Each 6 peptide subunit consists of a Y-shaped pair of triple peptide helices joined at the stem and ending in a globular non-helical head. The 80-amino acid helical component of each triple peptide contain many Gly-X-Y sequences, where X and Y are proline, isoleucine, or hydroxylysine; they, therefore, strongly resemble collagen fibr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin M
Immunoglobulin M (IgM) is the largest of several isotypes of antibodies (also known as immunoglobulin) that are produced by vertebrates. IgM is the first antibody to appear in the response to initial exposure to an antigen; causing it to also be called an acute phase antibody. In humans and other mammals that have been studied, plasmablasts in the spleen are the main source of specific IgM production. History In 1937, an antibody was observed in horses hyper-immunized with pneumococcus polysaccharide that was much larger in size than the typical rabbit γ-globulin, with a molecular weight of 990,000 daltons. In accordance with its larger size, the new antibody was originally referred to as γ-macroglobulin, and subsequently termed IgM—M for “macro”. The V domains of normal immunoglobulin are highly heterogeneous, reflecting their role in protecting against the great variety of infectious microbes, and this heterogeneity impeded detailed structural analysis of IgM. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibody-dependent Cellular Cytotoxicity
Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system kills a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection. ADCC is independent of the immune complement system that also lyses targets but does not require any other cell. ADCC requires an effector cell which classically is known to be natural killer (NK) cells that typically interact with immunoglobulin G (IgG) antibodies. However, macrophages, neutrophils and eosinophils can also mediate ADCC, such as eosinophils killing certain parasitic worms known as helminths via IgE antibodies. In general, ADCC has typically been described as the immune response to antibody-coated cells leading ultimately to the l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conformational Change
In biochemistry, a conformational change is a change in the shape of a macromolecule, often induced by environmental factors. A macromolecule is usually flexible and dynamic. Its shape can change in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a ''conformational change''. Factors that may induce such changes include temperature, pH, voltage, light in chromophores, concentration of ions, phosphorylation, or the binding of a ligand. Transitions between these states occur on a variety of length scales (tenths of Å to nm) and time scales (ns to s), and have been linked to functionally relevant phenomena such as allosteric signaling and enzyme catalysis. Laboratory analysis Many biophysical techniques such as crystallography, NMR, electron paramagnetic resonance (EPR) using spin label techniques, circular dichroism (CD), hydrogen exchange, and FRET can be used to study macromo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |