|
Mechanochemistry
Mechanochemistry (or mechanical chemistry) is the initiation of chemical reactions by mechanical phenomena. Mechanochemistry thus represents a fourth way to cause chemical reactions, complementing thermal reactions in fluids, photochemistry, and electrochemistry. Conventionally mechanochemistry focuses on the transformations of covalent bonds by mechanical force. Not covered by the topic are many phenomena: phase transitions, dynamics of biomolecules (docking, folding), and sonochemistry. Mechanochemistry is not the same as mechanosynthesis, which refers specifically to the machine-controlled construction of complex molecular products. In natural environments, mechanochemical reactions are frequently induced by physical processes such as earthquakes, glacier movement or hydraulic action of rivers or waves. In extreme environments such as subglacial lakes, hydrogen generated by mechnochemical reactions involving crushed silicate rocks and water can support methanogenic microbial co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanosynthesis
Mechanosynthesis is a term for hypothetical chemical syntheses in which reaction outcomes are determined by the use of mechanical constraints to direct reactive molecules to specific molecular sites. There are presently no non-biological chemical syntheses which achieve this aim. Some atomic placement has been achieved with scanning tunnelling microscopes. Introduction In conventional chemical synthesis or chemosynthesis, reactive molecules encounter one another through random thermal motion in a liquid or vapor. In a hypothesized process of mechanosynthesis, reactive molecules would be attached to molecular mechanical systems, and their encounters would result from mechanical motions bringing them together in planned sequences, positions, and orientations. It is envisioned that mechanosynthesis would avoid unwanted reactions by keeping potential reactants apart, and would strongly favor desired reactions by holding reactants together in optimal orientations for many molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Embryonic Differentiation Waves
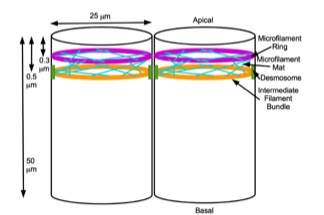
A mechanochemical based model for primary neural induction was first proposed in 1985 by Brodland and Gordon. They proposed that there is a mechanically sensitive bistable organelle made of microtubules and microfilaments in the apical ends of cells within cell sheets that are about to differentiate (that are competent) and these cells are under mechanical tension. The microtubules and microfilaments are in mechanical opposition in a proposed embryonic organelle they called the cell state splitter. Depending on where the cell is within a sheet, the tension will be resolved by either the apical end contracting or the apical end expanding. The resolution will begin at one point and spread over the rest of the tissue limited by other mechanical forces at boundaries. An actual physical wave of contraction has been found which traverses the presumptive neural epithelium of the developing salamander, the axolotl (''Ambystoma mexicanum''). The contraction wave's trajectory was more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving via an electronically-conducting phase (typically an external electrical circuit, but not necessarily, as in electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic species in a solution). When a chemical reaction is driven by an electrical potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some stag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanoluminescence
Mechanoluminescence is light emission resulting from any mechanical action on a solid. It can be produced through ultrasound, or through other means. * Fractoluminescence is caused by stress that results in the formation of fractures. * Piezoluminescence is caused by pressure that results only in elastic deformation. * Triboluminescence is nominally caused by rubbing, but actually because of resulting fractoluminescence, so is often used as a synonym. * Sonoluminescence is the emission of short bursts of light from imploding bubbles in a liquid when excited by sound. * Electrochemiluminescence is the emission induced by an electrochemical stimulus. See also *List of light sources This is a list of sources of light, the visible part of the electromagnetic spectrum. Light sources produce photons from another energy source, such as heat, chemical reactions, or conversion of mass or a different frequency of electromagnetic ener ... References {{reflist External links Ultrasou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Nitride
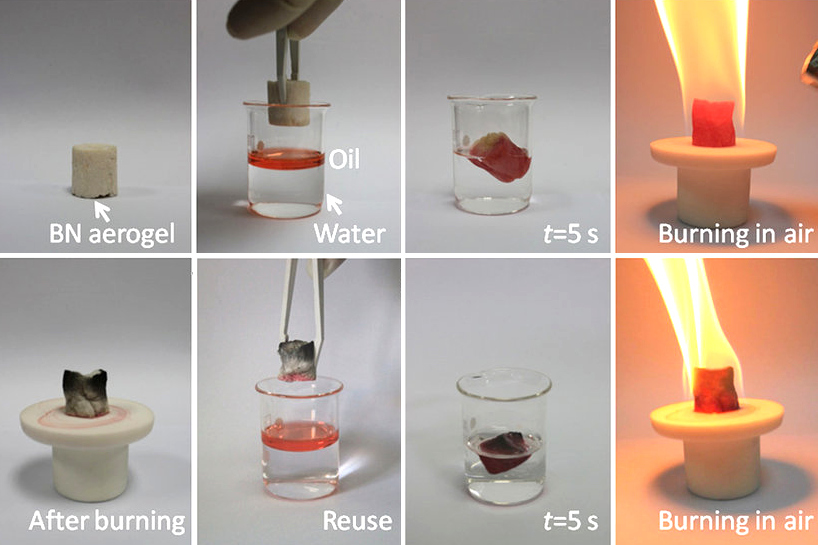
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. Structure Boron nitride exists in multiple forms that differ in the arrangement of the boron and nitrogen atoms, giving rise to var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodate
An iodate is the polyatomic anion with the formula . It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. Iodate salts are often colorless. They are the salts of iodic acid. Structure Iodate is pyramidal in structure. The O–I–O angles range from 97° to 105°, somewhat smaller than the O–Cl–O angles in chlorate. Reactions Redox Iodate is one of several oxyanions of iodine, and has an oxidation number of +5. It participates in several redox reactions, such as the iodine clock reaction. Iodate show no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate. Iodate is reduced by sulfite: :6HSO3- + 2IO3- -> 2I- + 6HSO4- Iodate oxidizes iodide: :5I- + IO3- + 3H2SO4 -> 3I2 + 3H2O + 3SO4^2- Similarly, chlorate oxidizes iodide to iodate: :I- + ClO3- -> Cl- + IO3- Iodate is also obtained by reducing a periodate with a sulfide. The byproduct of the reaction is a sulfoxide. Acid-base Iod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphyne
Graphyne is an allotrope of carbon. Its structure is one-atom-thick planar sheets of sp and sp2-bonded carbon atoms arranged in crystal lattice. It can be seen as a lattice of benzene rings connected by acetylene bonds. The material is called graphyne-''n'' when benzene rings are connected by ''n'' sequential acetylene molecules, and graphdiyne for a particular case of ''n'' = 2 ( diacetylene links). Depending on the content of acetylene groups, graphyne can be considered a mixed hybridization, spk, where 1 < k < 2, and thus differs from the hybridization of (considered pure sp2) and (pure sp3). First-principles calculations showed that periodic graphyne structures and their |
Magnets
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, cobalt, etc. and attracts or repels other magnets. A permanent magnet is an object made from a material that is magnetized and creates its own persistent magnetic field. An everyday example is a refrigerator magnet used to hold notes on a refrigerator door. Materials that can be magnetized, which are also the ones that are strongly attracted to a magnet, are called ferromagnetic (or ferrimagnetic). These include the elements iron, nickel and cobalt and their alloys, some alloys of rare-earth metals, and some naturally occurring minerals such as lodestone. Although ferromagnetic (and ferrimagnetic) materials are the only ones attracted to a magnet strongly enough to be commonly considered magnetic, all other substances respond weakly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. Synthesis Hydrazine, organohydrazines, and 1,1-diorganohydrazines react with aldehydes and ketones to give hydrazones. : Phenylhydrazine reacts with reducing sugars to form hydrazones known as osazones, which was developed by German chemist Emil Fischer as a test to differentiate monosaccharides. Uses Hydrazones are the basis for various analyses of ketones and aldehydes. For example, dinitrophenylhydrazine coated onto a silica sorbent is the basis of an adsorption cartridge. The hydrazones are then eluted and analyzed by HPLC using a UV detector. The compound carbonyl cyanide-''p''-trifluoromethoxyphenylhydrazone (abbreviated as FCCP) is used to uncouple ATP synthesis and reduction of oxygen in oxidative phosphorylation in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |