|
Moganite
Moganite is a tectosilicate mineral with the chemical formula Si O2 (silicon dioxide) that was discovered in 1976. It was initially described as a new form of silica from specimens found in the Barranco de Medio Almud, in the municipality of Mogán on the island of Gran Canaria, in the Canary Islands (Spain), receiving in a later work the name derived from this locality. In 1994 the International Mineralogical Association decided to disapprove it as a valid mineral, since it was considered indistinguishable from quartz. Subsequent studies allowed the IMA to rectify it in 1999, accepting it as a mineral species. It has the same chemical composition as quartz, but a different crystal structure. This mineral has been mainly found in dry locales such as Gran Canaria and Lake Magadi. It has been reported from a variety of locations in Europe, India and the United States. Physically, it has a Mohs hardness of about 6, a dull luster and appears as a semitransparent gray in color. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Silica Polymorphs
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical formula of Silicon dioxide, SiO2. Quartz is, therefore, classified structurally as a Silicate mineral#Tectosilicates, framework silicate mineral and compositionally as an oxide mineral. Quartz is the second most abundant mineral in Earth's continental crust, behind feldspar. Quartz exists in two forms, the normal α-quartz and the high-temperature β-quartz, both of which are chiral. The transformation from α-quartz to β-quartz takes place abruptly at . Since the transformation is accompanied by a significant change in volume, it can easily induce microfracturing of ceramics or rocks passing through this temperature threshold. There are many different varieties of quartz, several of which are classifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Agate
Agate ( ) is a banded variety of chalcedony. Agate stones are characterized by alternating bands of different colored chalcedony and sometimes include macroscopic quartz. They are common in nature and can be found globally in a large number of different varieties. There are some varieties of chalcedony without bands that are commonly called agate ( moss agate, fire agate, etc.); however, these are more properly classified solely as varieties of chalcedony. Agates are primarily formed as nodules within volcanic rock, but they can also form in veins or in sedimentary rock. Agate has been popular as a gemstone in jewelry for thousands of years, and today it is also popular as a collector's stone. Some duller agates sold commercially are artificially dyed to enhance their color. Etymology Agate was given its name by Theophrastus, a Greek philosopher and naturalist. He discovered the stone c. 350 BCE along the shoreline of the River Achates (), now the Dirillo River, on the Italian ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Oxide Minerals
The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal alloys. The hydroxide-bearing minerals are typically included in the oxide class. Minerals with complex anion groups such as the Silicate mineral, silicates, Sulfate mineral, sulfates, carbonate mineral, carbonates and Phosphate mineral, phosphates are classed separately. Simple oxides *XO form **Periclase group ***Periclase ***Manganosite **Zincite group ***Zincite ***Bromellite ***Tenorite ***Litharge * form **Cuprite **Ice * form **Hematite group ***Corundum ***Hematite ***Ilmenite * form **Rutile group ***Rutile ***Pyrolusite ***Cassiterite **Baddeleyite **Uraninite **Thorianite * form **Spinel group ***Spinel ***Gahnite ***Magnetite ***Franklinite ***Chromite **Chrysoberyl **Columbite *Hydroxide subgroup: **Brucite **Manganite **Romanèchite **Goethite group: ***Diaspore ***Goethite Nickel–Strunz class 4: oxides Internationa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Minerals In Space Group 15
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral or may be an aggregate of two or more different types of minerals, spacially segregated into distinct phases. So ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Monoclinic Minerals
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three Vector (geometric), vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism (geometry), prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. The length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal class ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Dehydroxylation
In chemistry, hydroxylation refers to the installation of a hydroxyl group () into an organic compound. Hydroxylations generate alcohols and phenols, which are very common functional groups. Hydroxylation confers some degree of water-solubility. Hydroxylation of a hydrocarbon is an oxidation, thus a step in degradation. Biological hydroxylation In biochemistry, hydroxylation reactions are often facilitated by enzymes called hydroxylases. These enzymes insert an O atom into a bond. Typical stoichiometries for the hydroxylation of a generic hydrocarbon are these: : : Since itself is a slow and unselective hydroxylating agent, catalysts are required to accelerate the pace of the process and to introduce selectivity. Hydroxylation is often the first step in the degradation of organic compounds in air. Hydroxylation is important in detoxification since it converts lipophilic compounds into water-soluble (hydrophilic) products that are more readily removed by the kidneys or liver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Dehydration
In physiology, dehydration is a lack of total body water that disrupts metabolic processes. It occurs when free water loss exceeds intake, often resulting from excessive sweating, health conditions, or inadequate consumption of water. Mild dehydration can also be caused by immersion diuresis, which may increase risk of decompression sickness in divers. Most people can tolerate a 3-4% decrease in total body water without difficulty or adverse health effects. A 5-8% decrease can cause fatigue and dizziness. Loss of over 10% of total body water can cause physical and mental deterioration, accompanied by severe thirst. Death occurs with a 15 and 25% loss of body water.Ashcroft F, Life Without Water in Life at the Extremes. Berkeley and Los Angeles, 2000, 134-138. Mild dehydration usually resolves with oral rehydration, but severe cases may need intravenous fluids. Dehydration can cause hypernatremia (high levels of sodium ions in the blood). This is distinct from hypovolemia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
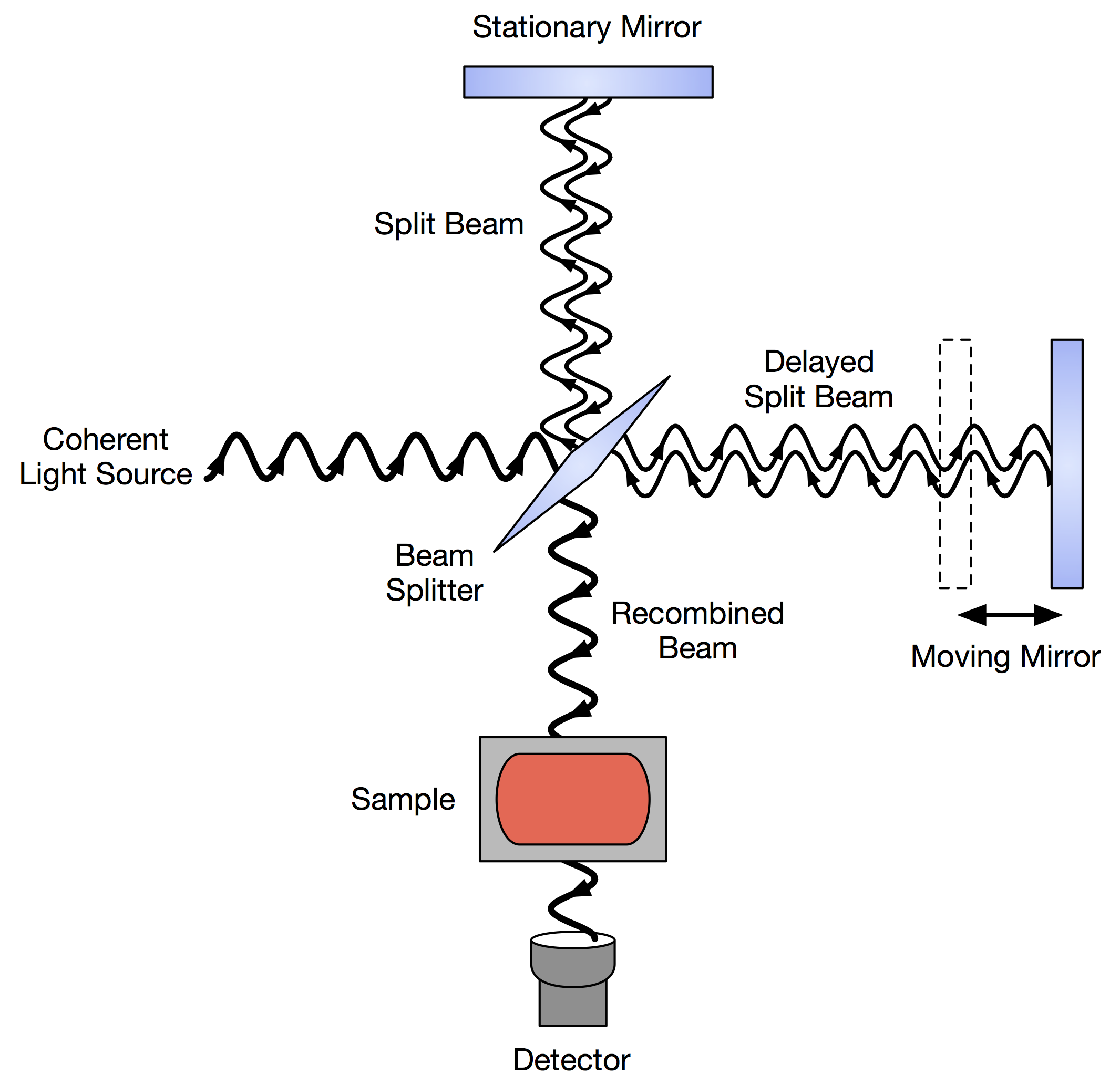
Fourier Transform Infrared Spectroscopy
Fourier transform infrared spectroscopy (FTIR) is a technique used to obtain an infrared Electromagnetic spectrum, spectrum of Absorption (electromagnetic radiation), absorption or Emission (electromagnetic radiation), emission of a solid, liquid, or gas. An FTIR spectrometer simultaneously collects high-resolution spectral data over a wide spectral range. This confers a significant advantage over a Dispersion (optics), dispersive spectrometer, which measures intensity over a narrow range of wavelengths at a time. The term ''Fourier transform infrared spectroscopy'' originates from the fact that a Fourier transform (a mathematical process) is required to convert the raw data into the actual spectrum. Conceptual introduction The goal of absorption spectroscopy techniques (FTIR, Ultraviolet-visible spectroscopy, ultraviolet-visible ("UV-vis") spectroscopy, etc.) is to measure how much light a sample absorbs at each wavelength. The most straightforward way to do this, the "dispe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phase Transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic State of matter, states of matter: solid, liquid, and gas, and in rare cases, plasma (physics), plasma. A phase of a thermodynamic system and the states of matter have uniform physical property, physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |