|
Microwave Chemistry
Microwave chemistry is the science of applying microwave radiation to chemical reactions. Microwaves act as high frequency electric fields and will generally heat any material containing mobile electric charges, such as polar molecules in a solvent or conducting ions in a solid. Microwave heating occurs primarily through two mechanisms: dipolar polarization and ionic conduction. Polar solvents because their dipole moments attempt to realign with the oscillating electric field, creating molecular friction and dielectric loss. The phase difference between the dipole orientation and the alternating field leads to energy dissipation as heat. Semiconducting and conducting samples heat when ions or electrons within them form an electric current and energy is lost due to the electrical resistance of the material .Commercial microwave systems typically operate at a frequency of 2.45 GHz, which allows effective energy transfer to polar molecules without quantum mechanical resonance effects. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microwave
Microwave is a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves. Its wavelength ranges from about one meter to one millimeter, corresponding to frequency, frequencies between 300 MHz and 300 GHz, broadly construed. A more common definition in radio-frequency engineering is the range between 1 and 100 GHz (wavelengths between 30 cm and 3 mm), or between 1 and 3000 GHz (30 cm and 0.1 mm). In all cases, microwaves include the entire super high frequency, super high frequency (SHF) band (3 to 30 GHz, or 10 to 1 cm) at minimum. The boundaries between far infrared, terahertz radiation, microwaves, and ultra-high-frequency (UHF) are fairly arbitrary and differ between different fields of study. The prefix ' in ''microwave'' indicates that microwaves are small (having shorter wavelengths), compared to the radio waves used in prior radio technology. Frequencies in the micr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
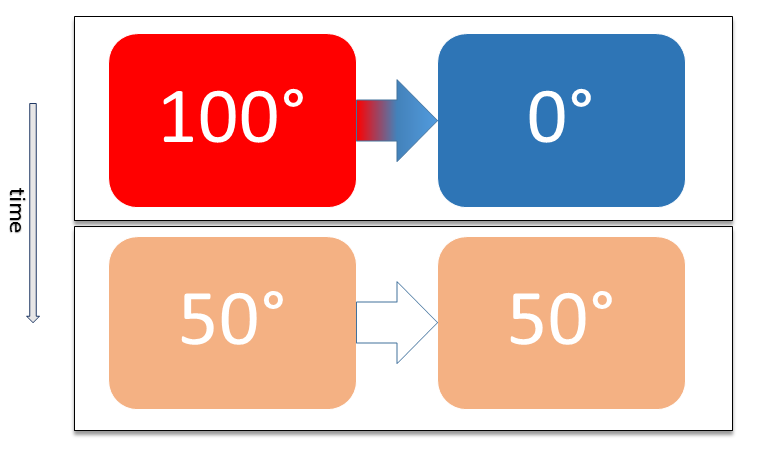
Thermal Equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in thermal equilibrium with itself if the temperature within the system is spatially uniform and temporally constant. Systems in thermodynamic equilibrium are always in thermal equilibrium, but the converse is not always true. If the connection between the systems allows transfer of energy as 'change in internal energy' but does not allow transfer of matter or transfer of energy as work, the two systems may reach thermal equilibrium without reaching thermodynamic equilibrium. Two varieties of thermal equilibrium Relation of thermal equilibrium between two thermally connected bodies The relation of thermal equilibrium is an instance of equilibrium between two bodies, which means that it refers to transfer through a selectively permeable par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterogeneous Catalysis
Heterogeneous catalysis is catalysis where the Phase (matter), phase of catalysts differs from that of the reagents or product (chemistry), products. The process contrasts with homogeneous catalysis where the reagents, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures (e.g., oil and water), or anywhere an interface is present. Heterogeneous catalysis typically involves solid phase catalysts and gas phase reactants. In this case, there is a cycle of molecular adsorption, reaction, and desorption occurring at the catalyst surface. Thermodynamics, mass transfer, and heat transfer influence the Reaction rate, rate (kinetics) of reaction. Heterogeneous catalysis is very important because it enables faster, large-scale production and the selective product formation. Approximately 35% of the world's GDP is influenced by catalysis. The production of 90% of chemicals (by volume) is assisted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-thermal Microwave Effect
Non-thermal microwave effects or specific microwave effects have been posited in order to explain unusual observations in microwave chemistry. The main effect of the absorption of microwaves by dielectric materials is a brief displacement in the permanent dipoles which causes rotational entropy. Since the frequency of the microwave energy is much faster than the electrons can absorb, the resultant energy can cause frictional heating of nearby atoms or molecules. If the material is rigid there will be no release of rotational energy, and therefore no heating. There are no "Non-thermal effects". If the material is not a dielectric material with dipoles or an ionic distribution, there is no interaction with microwaves and no heating. Non-thermal effects in liquids are almost certainly non-existent,Stuerga, D.; Gaillard, P. '' Journal of Microwave Power and Electromagnetic Energy'', 1996, 31, 101-113. http://jmpee.org/JMPEE_temp/31-2_bl/JMPEEA-31-2-Pg101.htmStuerga, D.; Gaillard, P. Journ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry Media Reaction
A dry media reaction or solid-state reaction or solventless reaction is a chemical reaction performed in the absence of a solvent. Dry media reactions have been developed in the wake of developments in microwave chemistry, and are a part of green chemistry. The drive for the development of dry media reactions in chemistry is: * economics (save money on solvents) * ease of purification (no solvent removal post-synthesis) * high reaction rate (due to high concentration of reactants) * environmentally friendly (solvent is not required), see green chemistry Drawbacks to overcome: * reactants should mix to a homogeneous system * high viscosity in reactant system * unsuitable for solvent assisted chemical reactions * problems with dissipating heat safely; risk of thermal runaway * side reactions accelerated * if reagents are solids, very high energy consumption from milling In one type of solventless reaction a liquid reactant is used neat, for instance the reaction of 1-bromonapht ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extraction (chemistry)
Extraction in chemistry is a separation process consisting of the separation of a substance from a Matrix (chemical analysis), matrix. The distribution of a solute between two phases is an Chemical equilibrium, equilibrium condition described by partition theory. This is based on exactly how the analyte moves from the initial solvent into the extracting solvent. The term ''washing'' may also be used to refer to an extraction in which impurities are extracted from the solvent containing the desired Chemical compound, compound. Types of extraction * Liquid–liquid extraction ** Acid-base extraction ** Supercritical fluid extraction * Solid-liquid extraction ** Solid-phase extraction ** Maceration ** Ultrasound assisted extraction, Ultrasound-assisted extraction ** Microwave-assisted extraction ** Heat reflux extraction ** Instant controlled pressure drop extraction (:fr:Détente instantanée contrôlée, Détente instantanée contrôlée) * Perstraction Laboratory applica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and polytetrafluoroethylene (PTFE). Chloroform was once used as an inhalational anesthetic between the 19th century and the first half of the 20th century. It is miscible with many solvents but it is only very slightly soluble in water (only 8 g/L at 20°C). Structure and name The molecule adopts a tetrahedral molecular geometry with C3v symmetry. The chloroform molecule can be viewed as a methane molecule with three hydrogen atoms replaced with three chlorine atoms, leaving a single hydrogen atom. The name "chloroform" is a portmanteau of ''terchloride'' (tertiary chloride, a trichloride) and ''formyle'', an obsolete name for the methylylidene radical (CH) derived from formic acid. Natural occurrence Many kinds of seaweed produce chlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transfer Catalyst
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of catalysis and can act through homogeneous catalysis or heterogeneous catalysis methods depending on the catalyst used. Ionic reactants are often soluble in an aqueous phase but insoluble in an organic phase in the absence of the phase-transfer catalyst. The catalyst functions like a detergent for solubilizing the salts into the organic phase. Phase-transfer catalysis refers to the acceleration of the reaction upon the addition of the phase-transfer catalyst. PTC is widely exploited industrially.Marc Halpern "Phase-Transfer Catalysis" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. Polyesters for example are prepared from acyl chlorides and bisphenol-A. Phosphothioate-based pesticides are generated by PTC-catalyzed alkylation of phosphothioates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polar Molecule
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. Molecules containing polar bonds have no molecular polarity if the bond dipoles cancel each other out by symmetry. Polar molecules interact through dipole-dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. Polarity of bonds Not all atoms attract electrons with the same force. The amount of "pull" an atom exerts on its electrons is called its electronegativity. Atoms with high electronegativitiessuch as fluorine, oxygen, and nitrogenexert a greater pull on electrons than atoms with lower electronegativities such as alkali metals and alkaline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binary
Binary may refer to: Science and technology Mathematics * Binary number, a representation of numbers using only two values (0 and 1) for each digit * Binary function, a function that takes two arguments * Binary operation, a mathematical operation that takes two arguments * Binary relation, a relation involving two elements * Finger binary, a system for counting in binary numbers on the fingers of human hands Computing * Binary code, the representation of text and data using only the digits 1 and 0 * Bit, or binary digit, the basic unit of information in computers * Binary file, composed of something other than human-readable text ** Executable, a type of binary file that contains machine code for the computer to execute * Binary tree, a computer tree data structure in which each node has at most two children * Binary-coded decimal, a method for encoding for decimal digits in binary sequences Astronomy * Binary star, a star system with two stars in it * Binary planet, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pelletizing
Pelletizing is the process of compressing or molding a material into the shape of a pellet. A wide range of different materials are pelletized including chemicals, iron ore, animal compound feed, plastics ( nurdles), waste materials, and more. The process is considered an excellent option for the storage and transport of said materials. The technology is widely used in the powder metallurgy engineering and medicine industries. Pelletizing of iron ore Edward W Davis of the University of Minnesota is credited for devising the process of pelletizing iron ore. Pelletizing iron ore is undertaken due to the excellent physical and metallurgical properties of iron ore pellets. Iron ore pellets are spheres of typically to be used as raw material for blast furnaces. They typically contain 64–72% Fe and various additional material adjusting the chemical composition and the metallurgic properties of the pellets. Typically limestone, dolomite and olivine is added and Bentonite is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |