 |
Maximum Bubble Pressure Method
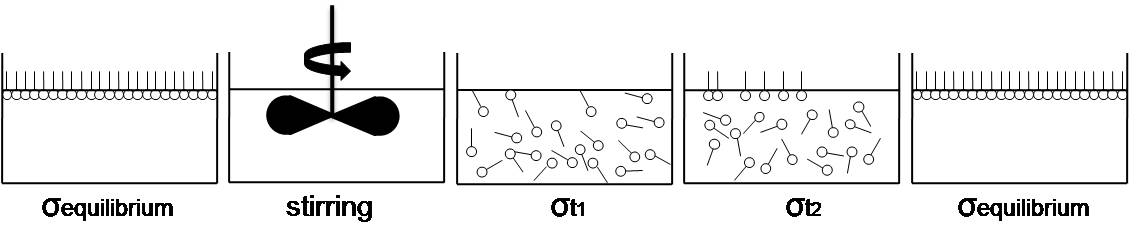
In physics, the maximum bubble pressure method, or in short bubble pressure method, is a technique to measure the surface tension of a liquid, with surfactants. __TOC__ Background When the liquid forms an interface with a gas phase, a molecule on the border has quite different Physical property, physical properties due to the unbalance of attracting forces by the neighboring molecules. At the Thermodynamic equilibrium, equilibrium state of the liquid, interior molecules are under the balanced forces with uniformly distributed adjacent molecules. However, relatively fewer number of molecules in the gas phase above the interface than Condensation, condensed liquid phase makes overall sum of forces applied to the surface molecule direct inside of the liquid and thus surface molecules tend to minimize their own surface area. Such an inequality of molecular forces induces continuous movement of molecules from the inside to the surface, which means the surface molecules has extra ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
Physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which relates to the order of nature, or, in other words, to the regular succession of events." It is one of the most fundamental scientific disciplines. "Physics is one of the most fundamental of the sciences. Scientists of all disciplines use the ideas of physics, including chemists who study the structure of molecules, paleontologists who try to reconstruct how dinosaurs walked, and climatologists who study how human activities affect the atmosphere and oceans. Physics is also the foundation of all engineering and technology. No engineer could design a flat-screen TV, an interplanetary spacecraft, or even a better mousetrap without first understanding the basic laws of physics. (...) You will come to see physics as a towering achievement of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Deformation (mechanics)
In physics and continuum mechanics, deformation is the change in the shape (geometry), shape or size of an object. It has dimension (physics), dimension of length with SI unit of metre (m). It is quantified as the residual displacement (geometry), displacement of particles in a non-rigid body, from an configuration to a configuration, excluding the body's average translation (physics), translation and rotation (its rigid transformation). A ''configuration'' is a set containing the position (geometry), positions of all particles of the body. A deformation can occur because of structural load, external loads, intrinsic activity (e.g. muscle contraction), body forces (such as gravity or electromagnetic forces), or changes in temperature, moisture content, or chemical reactions, etc. In a continuous body, a ''deformation field'' results from a Stress (physics), stress field due to applied forces or because of some changes in the conditions of the body. The relation between stre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Reduced Form
In statistics, and particularly in econometrics, the reduced form of a simultaneous equations model, system of equations is the result of solving the system for the endogenous variables. This gives the latter as functions of the exogenous variables, if any. In econometrics, the equations of a structural model (econometrics), structural form model are estimation theory, estimated in their theoretically given form, while an alternative approach to estimation is to first solve the theoretical equations for the endogenous variables to obtain reduced form equations, and then to estimate the reduced form equations. Let ''Y'' be the vector of the variables to be explained (endogeneous variables) by a statistical model and ''X'' be the vector of explanatory (exogeneous) variables. In addition let \varepsilon be a vector of error terms. Then the general expression of a structural form is f(Y, X, \varepsilon) = 0 , where ''f'' is a function, possibly from vectors to vectors in the case of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Young–Laplace Equation
In physics, the Young–Laplace equation () is an equation that describes the capillary pressure difference sustained across the interface between two static fluids, such as water and air, due to the phenomenon of surface tension or wall tension, although use of the latter is only applicable if assuming that the wall is very thin. The Young–Laplace equation relates the pressure difference to the shape of the surface or wall and it is fundamentally important in the study of static capillary surfaces. It is a statement of normal stress balance for static fluids meeting at an interface, where the interface is treated as a surface (zero thickness): \begin \Delta p &= -\gamma \nabla \cdot \hat n \\ &= -2\gamma H_f \\ &= -\gamma \left(\frac + \frac\right) \end where \Delta p is the Laplace pressure, the pressure difference across the fluid interface (the exterior pressure minus the interior pressure), \gamma is the surface tension (or wall tension), \hat n is the unit normal poi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and even by industry. Further, both spellings are often used ''within'' a particular industry or country. Industries in British English-speaking countries typically use the "gauge" spelling. is the pressure relative to the ambient pressure. Various #Units, units are used to express pressure. Some of these derive from a unit of force divided by a unit of area; the International System of Units, SI unit of pressure, the Pascal (unit), pascal (Pa), for example, is one newton (unit), newton per square metre (N/m2); similarly, the Pound (force), pound-force per square inch (Pound per square inch, psi, symbol lbf/in2) is the traditional unit of pressure in the imperial units, imperial and United States customary units, US customary systems. Pressure ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Tensiometer (surface Tension)
In surface science, a tensiometer is a measuring instrument used to measure the surface tension () of liquids or surfaces. Tensiometers are used in research and development laboratories to determine the surface tension of liquids like coatings, lacquers or adhesives. A further application field of tensiometers is the monitoring of industrial production processes like parts cleaning or electroplating. Types Goniometer/Tensiometer Surface scientists commonly use an optical goniometer/tensiometer to measure the surface tension and interfacial tension of a liquid using the pendant or sessile drop methods. A drop is produced and captured using a CCD camera. The drop profile is subsequently extracted, and sophisticated software routines then fit the theoretical Young-Laplace equation to the experimental drop profile. The surface tension can then be calculated from the fitted parameters. Unlike other methods, this technique requires only a small amount of liquid making it suitable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Molecular Structure
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of a molecule, i.e. they can be understood as approximately local and hence transferable properties. Determination The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR, microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography, neutron diffraction and electron diffraction can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Hydrophobe
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. The term ''hydrophobic''—which comes from the Ancient Greek (), "having a fear of water", constructed Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon. revised and augmented throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Hydrophile
A hydrophile is a molecule or other molecular entity that is intermolecular force, attracted to water molecules and tends to be dissolution (chemistry), dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press. In contrast, hydrophobes are not attracted to water and may seem to be repelled by it. Hygroscopics ''are'' attracted to water, but are not dissolved by water. Molecules A hydrophilic molecule or portion of a molecule is one whose interactions with water and other polar substances are more Thermodynamics, thermodynamically favorable than their interactions with oil or other hydrophobic solvents. They are typically charge-polarized and capable of hydrogen bonding. This makes these molecules soluble not only in water but also in Solvent#Solvent classifications, polar solvents. Hydrophilic molecules (and portions of molecules) can be contrasted with hydrophobic molecules (and portions of molecules). In some cases, both hydrophi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Measurement
Measurement is the quantification of attributes of an object or event, which can be used to compare with other objects or events. In other words, measurement is a process of determining how large or small a physical quantity is as compared to a basic reference quantity of the same kind. The scope and application of measurement are dependent on the context and discipline. In natural sciences and engineering, measurements do not apply to nominal properties of objects or events, which is consistent with the guidelines of the International Vocabulary of Metrology (VIM) published by the International Bureau of Weights and Measures (BIPM). However, in other fields such as statistics as well as the social and behavioural sciences, measurements can have multiple levels, which would include nominal, ordinal, interval and ratio scales. Measurement is a cornerstone of trade, science, technology and quantitative research in many disciplines. Historically, many measurement syste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |