|
Martensite
Martensite is a very hard form of steel crystalline structure. It is named after German metallurgist Adolf Martens. By analogy the term can also refer to any crystal structure that is formed by diffusionless transformation. Properties Martensite is formed in carbon steels by the rapid cooling ( quenching) of the austenite form of iron at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe3C). Austenite is gamma-phase iron (γ-Fe), a solid solution of iron and alloying elements. As a result of the quenching, the face-centered cubic austenite transforms to a highly strained body-centered tetragonal form called martensite that is supersaturated with carbon. The shear deformations that result produce a large number of dislocations, which is a primary strengthening mechanism of steels. The highest hardness of a pearlitic steel is 400 Brinell, whereas martensite can achieve 700&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tempering (metallurgy)
Tempering is a process of heat treating, which is used to increase the toughness of iron-based alloys. Tempering is usually performed after Hardening (metallurgy), hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point (thermodynamics), critical point for a certain period of time, then allowing it to cool in still air. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, while spring (device), springs are tempered at much higher temperatures. Introduction Tempering is a heat treatment technique applied to ferrous alloys, such as steel or cast iron, to achieve greater toughness by decreasing the hardness of the alloy. The reduction in hardness is usually accompanied by an increase in ductility, thereby decreasing the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Martensite
Martensite is a very hard form of steel crystalline structure. It is named after German metallurgist Adolf Martens. By analogy the term can also refer to any crystal structure that is formed by diffusionless transformation. Properties Martensite is formed in carbon steels by the rapid cooling ( quenching) of the austenite form of iron at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe3C). Austenite is gamma-phase iron (γ-Fe), a solid solution of iron and alloying elements. As a result of the quenching, the face-centered cubic austenite transforms to a highly strained body-centered tetragonal form called martensite that is supersaturated with carbon. The shear deformations that result produce a large number of dislocations, which is a primary strengthening mechanism of steels. The highest hardness of a pearlitic steel is 400 Brinell, whereas martensite can achieve 700&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
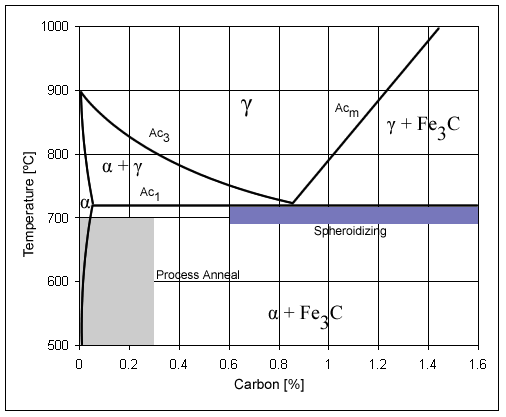
Austenite
Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902). It exists at room temperature in some stainless steels due to the presence of nickel stabilizing the austenite at lower temperatures. Allotrope of iron From alpha iron undergoes a phase transition from body-centered cubic (BCC) to the face-centered cubic (FCC) configuration of gamma iron, also called austenite. This is similarly soft and ductile but can dissolve considerably more carbon (as much as 2.03% by mass at ). This gamma form of iron is present in the most commonly used type of stainless steel for making hospital and food-service equipment. Material Austenitiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Steel
Steel is an alloy of iron and carbon that demonstrates improved mechanical properties compared to the pure form of iron. Due to steel's high Young's modulus, elastic modulus, Yield (engineering), yield strength, Fracture, fracture strength and low raw material cost, steel is one of the most commonly manufactured materials in the world. Steel is used in structures (as concrete Rebar, reinforcing rods), in Bridge, bridges, infrastructure, Tool, tools, Ship, ships, Train, trains, Car, cars, Bicycle, bicycles, Machine, machines, Home appliance, electrical appliances, furniture, and Weapon, weapons. Iron is always the main element in steel, but other elements are used to produce various grades of steel demonstrating altered material, mechanical, and microstructural properties. Stainless steels, for example, typically contain 18% chromium and exhibit improved corrosion and Redox, oxidation resistance versus its carbon steel counterpart. Under atmospheric pressures, steels generally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
TRIP Steel
TRIP steel are a class of high-strength steel alloys typically used in naval and marine applications and in the automotive industry. TRIP stands for "Transformation induced plasticity," which implies a phase transformation in the material, typically when a stress is applied. These alloys are known to possess an outstanding combination of strength and ductility. Microstructure TRIP steels possess a microstructure consisting of austenite with sufficient thermodynamic instability such that transformation to martensite is achieved during loading or deformation. Many automotive TRIP steels possess retained austenite within a ferrite matrix, which may also contain hard phases like bainite and martensite. In the case of these alloys, the high silicon and carbon content of TRIP steels results in significant volume fractions of retained austenite in the final microstructure. TRIP steels use higher quantities of carbon than dual-phase steels to obtain sufficient carbon content for stabilizi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Diffusionless Transformation
A diffusionless transformation, commonly known as displacive transformation, denotes solid-state alterations in crystal structures that do not hinge on the diffusion of atoms across extensive distances. Rather, these transformations manifest as a result of synchronized shifts in atomic positions, wherein atoms undergo displacements of distances smaller than the spacing between adjacent atoms, all while preserving their relative arrangement. An example of such a phenomenon is the martensitic transformation, a notable occurrence observed in the context of steel materials. The term "martensite" was originally coined to describe the rigid and finely dispersed constituent that emerges in steels subjected to rapid cooling. Subsequent investigations revealed that materials beyond ferrous alloys, such as non-ferrous alloys and ceramics, can also undergo diffusionless transformations. Consequently, the term "martensite" has evolved to encompass the resultant product arising from such tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbon Steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states: * no minimum content is specified or required for chromium, cobalt, molybdenum, nickel, niobium, titanium, tungsten, vanadium, zirconium, or any other element to be added to obtain a desired alloying effect; * the specified minimum for copper does not exceed 0.40%; * or the specified maximum for any of the following elements does not exceed: manganese 1.65%; silicon 0.60%; and copper 0.60%. As the carbon content percentage rises, steel has the ability to become harder and stronger through heat treating; however, it becomes less ductile. Regardless of the heat treatment, a higher carbon content reduces weldability. In carbon steels, the higher carbon content lowers the melting point. The term may be used to reference steel that is not stainless steel; in this use carbon steel may include alloy st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Quench
In materials science, quenching is the rapid cooling of a workpiece in water, gas, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as phase transformations, from occurring. It does this by reducing the window of time during which these undesired reactions are both thermodynamically favorable and kinetically accessible; for instance, quenching can reduce the crystal grain size of both metallic and plastic materials, increasing their hardness. In metallurgy, quenching is most commonly used to harden steel by inducing a martensite transformation, where the steel must be rapidly cooled through its eutectoid point, the temperature at which austenite becomes unstable. Rapid cooling prevents the formation of cementite structure, instead forcibly dissolving carbon atoms in the ferrite lattice. In steel alloyed with metals such as nickel and manganese, the eutectoid tempe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have properties that differ from those of the pure elements from which they are made. The vast majority of metals used for commercial purposes are alloyed to improve their properties or behavior, such as increased strength, hardness or corrosion resistance. Metals may also be alloyed to reduce their overall cost, for instance alloys of gold and Copper(II) sulfate, copper. A typical example of an alloy is SAE 304 stainless steel, 304 grade stainless steel which is commonly used for kitchen utensils, pans, knives and forks. Sometime also known as 18/8, it as an alloy consisting broadly of 74% iron, 18% chromium and 8% nickel. The chromium and nickel alloying elements add strength and hardness to the majority iron element, but their main function is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Alloy Steel
Alloy steel is steel that is Alloy, alloyed with a variety of elements in amounts between 1.0% and 50% by weight, typically to improve its List of materials properties#Mechanical properties, mechanical properties. Types Alloy steels divide into two groups: low and high alloy. The boundary between the two is disputed. Smith and Hashemi define the difference at 4.0%, while Degarmo, ''et al.'', define it at 8.0%. Most alloy steels are low-alloy. The simplest steels are iron (Fe) alloyed with (0.1% to 1%) carbon (C) and nothing else (excepting slight impurities); these are called Carbon steel, carbon steels. However, alloy steel encompasses steels with additional (metal) alloying elements. Common alloyants include manganese (Mn) (the most common), nickel (Ni), chromium (Cr), molybdenum (Mo), vanadium (V), silicon (Si), and boron (B). Less common alloyants include Aluminium (Al), cobalt (Co), copper (Cu), cerium (Ce), niobium (Nb), titanium (Ti), tungsten (W), tin (Sn), zinc (Zn), le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |