|
Lume
Lume is a short term for the luminous phosphorescent glowing solution applied on watch dials. There are some people who "relume" watches, or replace faded lume. Formerly, lume consisted mostly of radium; however, radium is radioactive and has been mostly replaced on new watches by less bright, but less toxic compounds. Common pigments used in lume include the phosphorescent pigments zinc sulfide and strontium aluminate Strontium aluminate is an aluminate compound with the chemical formula (sometimes written as ). It is a pale yellow, monoclinic crystalline powder that is odourless and non-flammable. When activated with a suitable dopant (e.g. europium, written .... Use of zinc sulfide for safety related products dates back to the 1930s. However, the development of strontium oxide aluminate, with a luminance approximately 10 times greater than zinc sulfide, has relegated most zinc sulfide based products to the novelty category. Strontium oxide aluminate based pigments are now u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Super-LumiNova
Super-LumiNova is a brand name under which strontium aluminate–based non-radioactive and nontoxic photoluminescent or afterglow pigments for illuminating markings on watch dials, hands and bezels, etc. in the dark are marketed. This technology offers up to ten times higher brightness than previous zinc sulfide–based materials. Super-LumiNova is based on LumiNova pigments, invented in 1993 by Nemoto & Co., Ltd. of Japan as a safe replacement for radium-based luminous paints. Nemoto & Co. was founded in December 1941 as a luminous paint processing company and has supplied paint to the watch and clock industry for over 70 years. Besides being used in timepieces, Super-LumiNova is also marketed for application on: * Instruments: scales, dials, markings, indicators etc. * Scales: engravings, silkscreen-printing * Aviation instruments and markings * Jewelry * Safety- and emergency-panels, signs, markings * Aiming posts * Various other parts These types of phosphorescent pigmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Luminescent Paint Pigment Applied On A Diver’s Watch To Make It Readable In Low Light Conditions
Luminescence is spontaneous emission of light by a substance not resulting from heat; or "cold light". It is thus a form of cold-body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a substance as a result of heating. Historically, radioactivity was thought of as a form of "radio-luminescence", although it is today considered to be separate since it involves more than electromagnetic radiation. The dials, hands, scales, and signs of aviation and navigational instruments and markings are often coated with luminescent materials in a process known as "luminising". Types The following are types of luminescence: *Chemiluminescence, the emission of light as a result of a chemical reaction **Bioluminescence, a result of biochemical reactions in a living organism **Electrochemiluminescence, a result of an electrochemical reaction **Lyoluminesce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed. In a general sense, there is no distinct boundary between the emission times of fluorescence and phosphorescence (i.e.: if a substance glows under a black light it is generally considered fluorescent, and if it glows in the dark it is often simply called phosphorescent). In a modern, scientific sense, the phenomena can usually be classified by the three different mechanisms that produce the light, and the typical timescales during which those mechanisms emit light. Whereas fluorescent materials stop emit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Watch Dial
A watch is a portable timepiece intended to be carried or worn by a person. It is designed to keep a consistent movement despite the motions caused by the person's activities. A wristwatch is designed to be worn around the wrist, attached by a watch strap or other type of bracelet, including metal bands, leather straps or any other kind of bracelet. A pocket watch is designed for a person to carry in a pocket, often attached to a chain. Watches were developed in the 17th century from spring-powered clocks, which appeared as early as the 14th century. During most of its history the watch was a mechanical device, driven by clockwork, powered by winding a mainspring, and keeping time with an oscillating balance wheel. These are called '' mechanical watches''. In the 1960s the electronic ''quartz watch'' was invented, which was powered by a battery and kept time with a vibrating quartz crystal. By the 1980s the quartz watch had taken over most of the market from the mec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather than oxygen) upon exposure to air, forming a black surface layer of radium nitride (Ra3N2). All isotopes of radium are radioactive, the most stable isotope being radium-226 with a half-life of 1600 years. When radium decays, it emits ionizing radiation as a by-product, which can excite fluorescent chemicals and cause radioluminescence. Radium, in the form of radium chloride, was discovered by Marie and Pierre Curie in 1898 from ore mined at Jáchymov. They extracted the radium compound from uraninite and published the discovery at the French Academy of Sciences five days later. Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne through the electrolysis of radium chloride in 1911. In nature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay ( ), beta decay ( ), and gamma decay ( ), all of which involve emitting one or more particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force. A fourth type of common decay is electron capture, in which an unstable nucleus captures an inner electron from one of the electron shells. The loss of that electron from the shell results in a cascade of electrons dropping down to that lower shell resulting in emission of discrete X-rays from the transitions. A common example is iodine-125 commonly used in medical settings. Radioactive decay is a stochastic (i.e. random) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
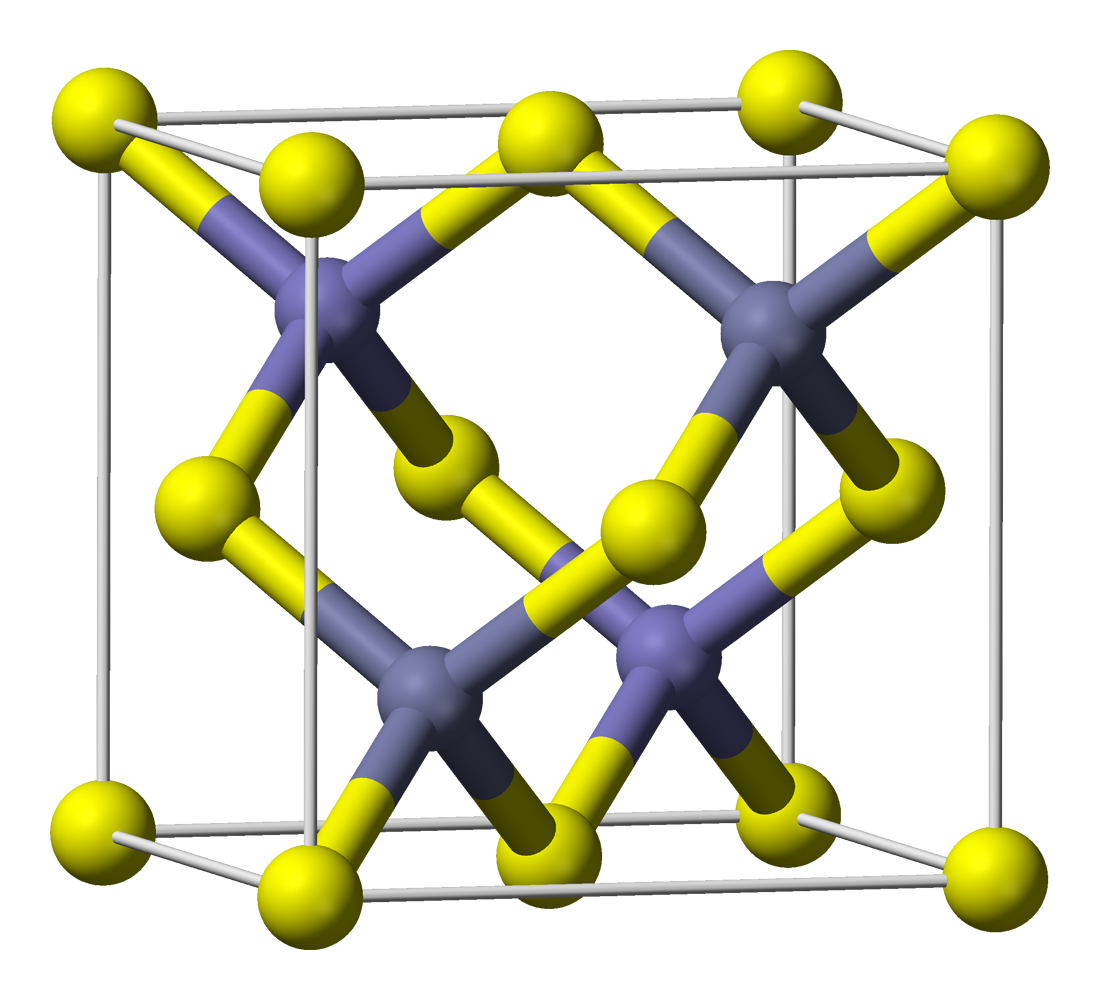
Zinc Sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. A tetragonal form is also known as the very rare mineral called polhemusite, with the formula . Appli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium Aluminate
Strontium aluminate is an aluminate compound with the chemical formula (sometimes written as ). It is a pale yellow, monoclinic crystalline powder that is odourless and non-flammable. When activated with a suitable dopant (e.g. europium, written as ), it acts as a photoluminescent phosphor with long persistence of phosphorescence. Strontium aluminates exist in a variety of other compositions including (monoclinic), (cubic), (hexagonal), and (orthorhombic). The different compositions cause different colours of light to be emitted. History Phosphorescent materials were discovered in the 1700s, and people have been studying them and making improvements over the centuries. The development of strontium aluminate pigments in 1993 was spurred on by the need to find a substitute for glow-in-the-dark materials with high luminance and long phosphorescence, especially those that used promethium. This led to the discovery by Yasumitsu Aoki (Nemoto & Co.) of materials with luminance appro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Luminescence
Luminescence is spontaneous emission of light by a substance not resulting from heat; or "cold light". It is thus a form of cold-body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a substance as a result of heating. Historically, radioactivity was thought of as a form of "radio-luminescence", although it is today considered to be separate since it involves more than electromagnetic radiation. The dials, hands, scales, and signs of aviation and navigational instruments and markings are often coated with luminescent materials in a process known as "luminising". Types The following are types of luminescence: * Chemiluminescence, the emission of light as a result of a chemical reaction ** Bioluminescence, a result of biochemical reactions in a living organism **Electrochemiluminescence, a result of an electrochemical reaction **Lyolum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |