|
Isobar (nuclide)
Isobars are atoms (nuclides) of different chemical elements that have the same number of nucleons. Correspondingly, isobars differ in atomic number (or number of protons) but have the same mass number. An example of a series of isobars is 40S, 40Cl, 40Ar, 40K, and 40Ca. While the nuclei of these nuclides all contain 40 nucleons, they contain varying numbers of protons and neutrons. The term "isobars" (originally "isobares") for nuclides was suggested by Alfred Walter Stewart in 1918. It is derived from the Greek word ''isos'', meaning "equal" and ''baros'', meaning "weight". Mass The same mass number implies neither the same mass of nuclei, nor equal atomic masses of corresponding nuclides. From the Weizsäcker formula for the mass of a nucleus: : m(A,Z) = Z m_p + N m_n - a_ A + a_ A^ + a_ \frac + a_ \frac - \delta(A,Z) where mass number equals to the sum of atomic number and number of neutrons , and , , , , , are constants, one can see that t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 26,634 (uranium atomic r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Binding Energy
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is always a positive number, as the nucleus must gain energy for the nucleons to move apart from each other. Nucleons are attracted to each other by the strong nuclear force. In theoretical nuclear physics, the nuclear binding energy is considered a negative number. In this context it represents the energy of the nucleus relative to the energy of the constituent nucleons when they are infinitely far apart. Both the experimental and theoretical views are equivalent, with slightly different emphasis on what the binding energy means. The mass of an atomic nucleus is less than the sum of the individual masses of the free constituent protons and neutrons. The difference in mass can be calculated by the Einstein equation, , where ''E'' is the nuclear b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Subtraction
Subtraction is an arithmetic operation that represents the operation of removing objects from a collection. Subtraction is signified by the minus sign, . For example, in the adjacent picture, there are peaches—meaning 5 peaches with 2 taken away, resulting in a total of 3 peaches. Therefore, the ''difference'' of 5 and 2 is 3; that is, . While primarily associated with natural numbers in arithmetic, subtraction can also represent removing or decreasing physical and abstract quantities using different kinds of objects including negative numbers, fractions, irrational numbers, vectors, decimals, functions, and matrices. Subtraction follows several important patterns. It is anticommutative, meaning that changing the order changes the sign of the answer. It is also not associative, meaning that when one subtracts more than two numbers, the order in which subtraction is performed matters. Because is the additive identity, subtraction of it does not change a number. Subtrac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Even Number
In mathematics, parity is the property of an integer of whether it is even or odd. An integer is even if it is a multiple of two, and odd if it is not.. For example, −4, 0, 82 are even because \begin -2 \cdot 2 &= -4 \\ 0 \cdot 2 &= 0 \\ 41 \cdot 2 &= 82 \end By contrast, −3, 5, 7, 21 are odd numbers. The above definition of parity applies only to integer numbers, hence it cannot be applied to numbers like 1/2 or 4.201. See the section "Higher mathematics" below for some extensions of the notion of parity to a larger class of "numbers" or in other more general settings. Even and odd numbers have opposite parities, e.g., 22 (even number) and 13 (odd number) have opposite parities. In particular, the parity of zero is even. Any two consecutive integers have opposite parity. A number (i.e., integer) expressed in the decimal numeral system is even or odd according to whether its last digit is even or odd. That is, if the last digit is 1, 3, 5, 7, or 9, then it is odd; otherw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Product
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps ( decay chain). For example, 238U decays to 234Th which decays to 234mPa which decays, and so on, to 206Pb (which is stable): : \ce \overbrace^\ce left, upThe decay chain from lead-212 down to lead-208, showing the intermediate decay products In this example: * 234Th, 234mPa,...,206Pb are the decay products of 238U. * 234Th is the daughter of the parent 238U. * 234mPa (234 metastable) is the granddaughter of 238U. These might also be referred to as the daughter products of 238U. (''Depleted Uranium'' — authors: Naomi H. Harley, Ernest C. Foulkes, Lee H. H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Mode
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay ( ), beta decay ( ), and gamma decay ( ), all of which involve emitting one or more particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force. A fourth type of common decay is electron capture, in which an unstable nucleus captures an inner electron from one of the electron shells. The loss of that electron from the shell results in a cascade of electrons dropping down to that lower shell resulting in emission of discrete X-rays from the transitions. A common example is iodine-125 commonly used in medical settings. Radioactive decay is a stochastic (i.e. random) pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positron Decay
Positron emission, beta plus decay, or β+ decay is a subtype of radioactive decay called beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino (). Positron emission is mediated by the weak force. The positron is a type of beta particle (β+), the other beta particle being the electron (β−) emitted from the β− decay of a nucleus. An example of positron emission (β+ decay) is shown with magnesium-23 decaying into sodium-23: : → + + Because positron emission decreases proton number relative to neutron number, positron decay happens typically in large "proton-rich" radionuclides. Positron decay results in nuclear transmutation, changing an atom of one chemical element into an atom of an element with an atomic number that is less by one unit. Positron emission occurs only very rarely naturally on earth, when induced by a cosmic ray or from one in a hundred thousand decays of pot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
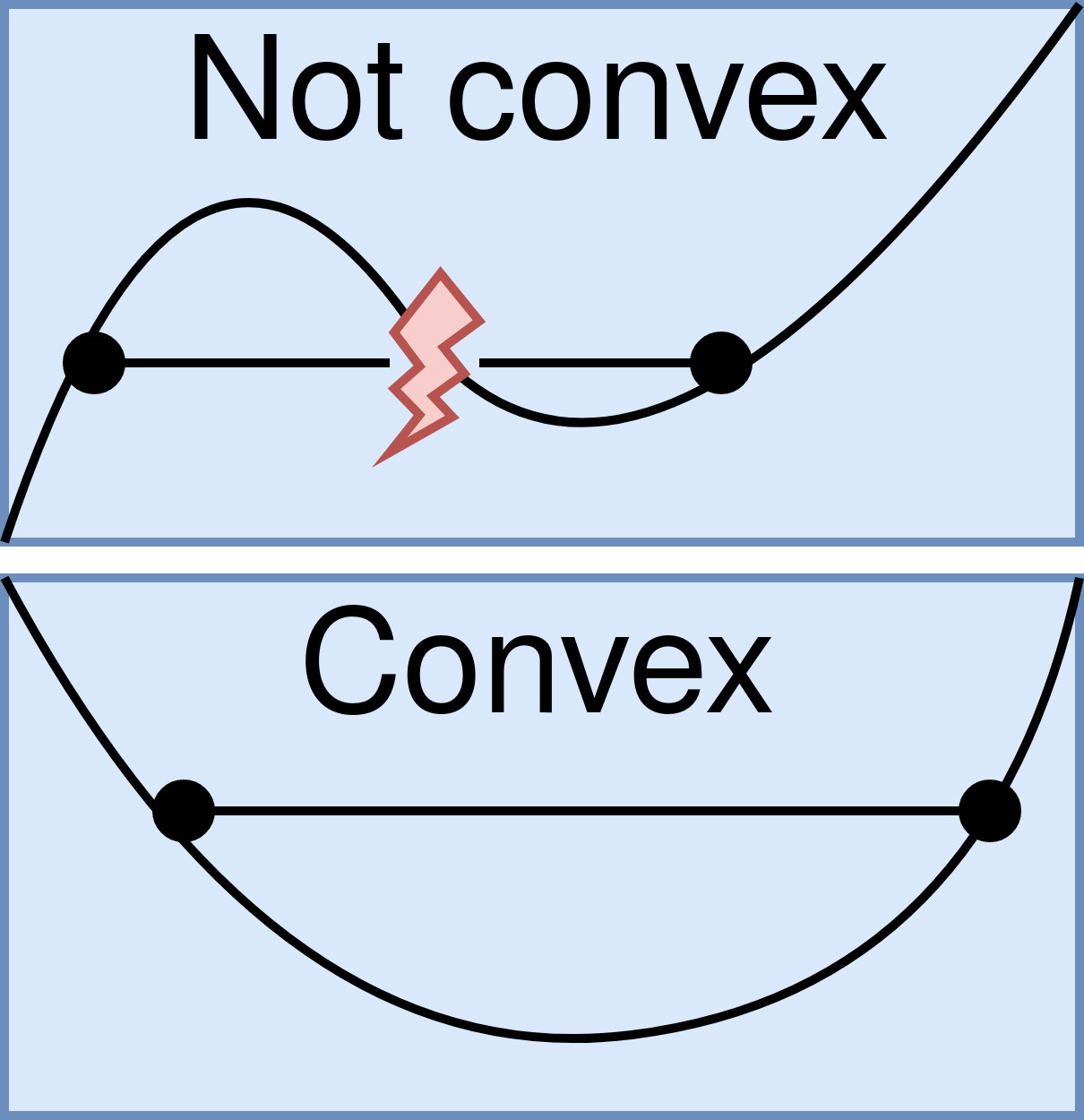
Convex Function
In mathematics, a real-valued function is called convex if the line segment between any two points on the graph of the function lies above the graph between the two points. Equivalently, a function is convex if its epigraph (the set of points on or above the graph of the function) is a convex set. A twice-differentiable function of a single variable is convex if and only if its second derivative is nonnegative on its entire domain. Well-known examples of convex functions of a single variable include the quadratic function x^2 and the exponential function e^x. In simple terms, a convex function refers to a function whose graph is shaped like a cup \cup, while a concave function's graph is shaped like a cap \cap. Convex functions play an important role in many areas of mathematics. They are especially important in the study of optimization problems where they are distinguished by a number of convenient properties. For instance, a strictly convex function on an open set has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |