|
Ivacaftor
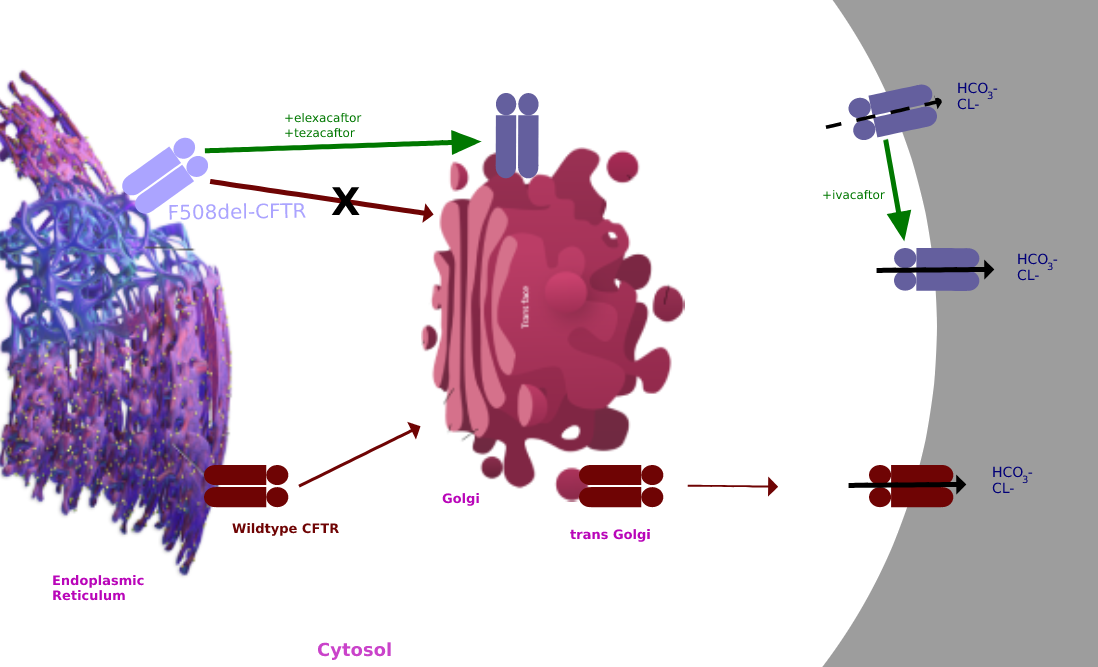
Ivacaftor is a medication used to treat cystic fibrosis in people with certain mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (primarily the G551D mutation), who account for 4–5% cases of cystic fibrosis. It is also included in combination medications, lumacaftor/ivacaftor, tezacaftor/ivacaftor, and elexacaftor/tezacaftor/ivacaftor which are used to treat people with cystic fibrosis. Ivacaftor was developed by Vertex Pharmaceuticals in conjunction with the Cystic Fibrosis Foundation and is the first medication that treats the underlying cause rather than the symptoms of the disease. It was approved by the U.S. Food and Drug Administration (FDA) in January 2012. It is one of the most expensive drugs, costing over per year, which has led to criticism of the high cost. The combination drug lumacaftor/ivacaftor was approved by the FDA in July 2015. Cystic fibrosis is caused by any one of several defects in the CFTR protein, which regulates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trikafta
Elexacaftor/tezacaftor/ivacaftor, sold under the brand names Trikafta and Kaftrio, is a fixed-dose combination medication used to treat cystic fibrosis. Elexacaftor/tezacaftor/ivacaftor is composed of a combination of ivacaftor, a chloride channel opener, and elexacaftor and tezacaftor, CFTR modulators. It is approved for use in the United States for people aged two years and older who have cystic fibrosis with a Cystic fibrosis transmembrane conductance regulator#DeltaF508, F508del mutation or other mutations in the CFTR gene. It is also approved for use in Canada, the European Union, and Australia. Medical uses The combination is indicated for the treatment of people aged two years and older who have cystic fibrosis with a F508del mutation or other mutations in the CFTR gene. Side effects The most common side effects affecting more than 5% of patients are headache, upper respiratory tract infection, abdominal pain, diarrhea, rash, alanine aminotransferase increase, nas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lumacaftor/ivacaftor
Lumacaftor/ivacaftor, sold under the brand name Orkambi among others, is a combination of lumacaftor and ivacaftor used to treat people with cystic fibrosis who have two copies of the F508del mutation. It is unclear if it is useful in cystic fibrosis due to other causes. It is taken by mouth. Common side effects include shortness of breath, nausea, diarrhea, feeling tired, hearing problems, and rash. Severe side effects may include liver problems and cataracts. Ivacaftor increases the activity of the CFTR protein, while lumacaftor improves protein folding of the CFTR protein. It was approved for medical use in the United States in 2015, and in Canada in 2016. In the United States it costs more than a month as of 2018. While its use was not recommended in the United Kingdom as of 2018, pricing was agreed upon in 2019 and it is expected to be covered by November of that year. Medical use The combination of lumacaftor/ivacaftor is used to treat people with cystic fibrosis wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tezacaftor
Tezacaftor is a medication used for the treatment of cystic fibrosis. It is available in fixed-dose combination medications. The combination of tezacaftor with ivacaftor (brand name Symdeko) was approved for medical use in the United States in February 2018, and in Canada in June 2018. As brand name Symkevi it was approved for medical use in the European Union in October 2018. The combination of tezacaftor with elexacaftor and ivacaftor (brand name Trikafta) was approved for medical use in the United States in October 2019, and in Canada in June 2021. As brand name Kaftrio it was approved for medical use in the European Union in August 2020. The combination of tezacaftor with vanzacaftor and deutivacaftor (brand name Alyftrek) was approved for medical use in the United States in December 2024. Mechanism of action Tezacaftor acts as a corrector to help the folding and presentation of the CFTR protein to the cell surface, which improves its function for individuals with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vertex Pharmaceuticals
Vertex Pharmaceuticals Incorporated is an American biopharmaceutical company based in Boston, Massachusetts. It was one of the first biotech firms to use an explicit strategy of rational drug design rather than combinatorial chemistry. It maintains headquarters in Boston, Massachusetts, and three research facilities, in San Diego, California, and Milton Park, Oxfordshire, England. History Vertex was founded in 1989 by Joshua Boger and Kevin J. Kinsella . to "transform the way serious diseases are treated." The company's beginnings were profiled by Barry Werth in the 1994 book '' The Billion-Dollar Molecule''. His 2014 book, ''The Antidote: Inside the World of New Pharma'', chronicled the company's subsequent development over the next two decades. By 2004, its product pipeline focused on viral infections, inflammatory and autoimmune disorders, and cancer. In 2009, the company had about 1,800 employees, including 1,200 in the Boston area. By 2019 there were about 2,500 empl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elexacaftor
Elexacaftor is a medication that acts as cystic fibrosis transmembrane conductance regulator (CFTR) corrector. It is available in a single pill with ivacaftor and tezacaftor; the fixed-dose combination, elexacaftor/tezacaftor/ivacaftor (brand name ''Trikafta''), is used to treat people with cystic fibrosis who are homozygous for the f508del mutation. This combination was approved for medical use in the United States in 2019. The fixed-dose combination elexacaftor/tezacaftor/ivacaftor (Kaftrio) was approved for medical use in the European Union The European Union (EU) is a supranational union, supranational political union, political and economic union of Member state of the European Union, member states that are Geography of the European Union, located primarily in Europe. The u ... in August 2020, for the treatment of cystic fibrosis. References External links * Cystic fibrosis Orphan drugs Acylsulfonamides {{respiratory-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystic Fibrosis
Cystic fibrosis (CF) is a genetic disorder inherited in an autosomal recessive manner that impairs the normal clearance of Sputum, mucus from the lungs, which facilitates the colonization and infection of the lungs by bacteria, notably ''Staphylococcus aureus''. CF is a rare genetic disorder that affects mostly the lungs, but also the pancreas, liver, kidneys, and intestine. The hallmark feature of CF is the accumulation of thick mucus in different organs. Long-term issues include Shortness of breath, difficulty breathing and coughing up mucus as a result of frequent pneumonia, lung infections. Other signs and symptoms may include Sinusitis, sinus infections, failure to thrive, poor growth, Steatorrhea, fatty stool, Nail clubbing, clubbing of the fingers and toes, and infertility in most males. Different people may have different degrees of symptoms. Cystic fibrosis is inherited in an autosomal recessive manner. It is caused by the presence of mutations in both copies (alleles) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
F508del
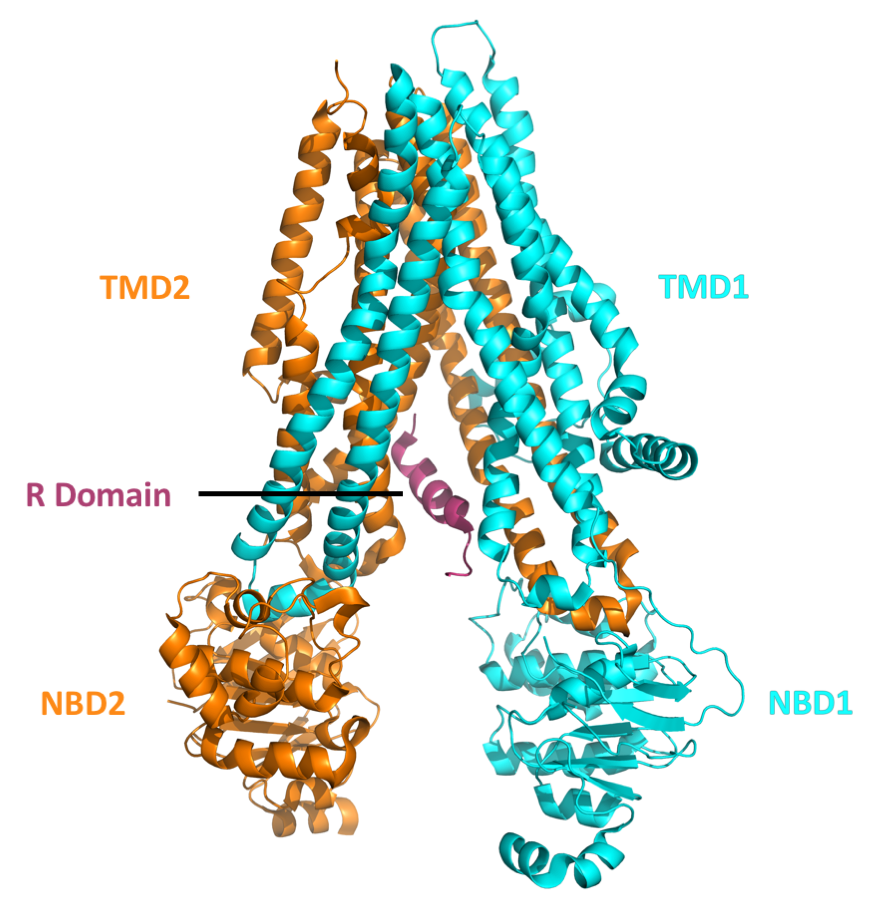
Cystic fibrosis transmembrane conductance regulator (CFTR) is a membrane protein and anion channel in vertebrates that is encoded by the ''CFTR'' gene. Geneticist Lap-Chee Tsui and his team identified the ''CFTR'' gene in 1989 as the gene linked with CF (cystic fibrosis). The ''CFTR'' gene codes for an ABC transporter-class ion channel protein that conducts chloride and bicarbonate ions across epithelial cell membranes. Mutations of the ''CFTR'' gene affecting anion channel function lead to dysregulation of epithelial lining fluid (mucus) transport in the lung, pancreas and other organs, resulting in cystic fibrosis. Complications include thickened mucus in the lungs with frequent respiratory infections, and pancreatic insufficiency giving rise to malnutrition and diabetes. These conditions lead to chronic disability and reduced life expectancy. In male patients, the progressive obstruction and destruction of the developing vas deferens (spermatic cord) and epididymis appear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ΔF508
Cystic fibrosis transmembrane conductance regulator (CFTR) is a membrane protein and anion channel in vertebrates that is encoded by the ''CFTR'' gene. Geneticist Lap-Chee Tsui and his team identified the ''CFTR'' gene in 1989 as the gene linked with CF (cystic fibrosis). The ''CFTR'' gene codes for an ABC transporter-class ion channel protein that conducts chloride and bicarbonate ions across epithelial cell membranes. Mutations of the ''CFTR'' gene affecting anion channel function lead to dysregulation of epithelial lining fluid (mucus) transport in the lung, pancreas and other organs, resulting in cystic fibrosis. Complications include thickened mucus in the lungs with frequent respiratory infections, and pancreatic insufficiency giving rise to malnutrition and diabetes. These conditions lead to chronic disability and reduced life expectancy. In male patients, the progressive obstruction and destruction of the developing vas deferens (spermatic cord) and epididymis appe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystic Fibrosis Foundation
The Cystic Fibrosis Foundation (CFF) is a 501(c)(3) non-profit organization in the United States established to provide the means to cure cystic fibrosis (CF) and ensure that those living with CF live long and productive lives. The Foundation provides information about cystic fibrosis and finances CF research that aims to improve the quality of life for people with the disease. The Foundation also engages in legislative lobbying for cystic fibrosis. History The Foundation was established in 1955 by a group of volunteers in Philadelphia, Pennsylvania. In addition to providing grants for research into cystic fibrosis and supporting clinical trials, the foundation promotes and accredits 115 specialized centers for treatment of individuals with cystic fibrosis. The Foundation has over 80 chapters and offices across the United States. Before it began using the current name, the organization was known as the "National Cystic Fibrosis Research Foundation". In 1989, scientists working ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystic Fibrosis Transmembrane Conductance Regulator
Cystic fibrosis transmembrane conductance regulator (CFTR) is a membrane protein and anion channel in vertebrates that is encoded by the ''CFTR'' gene. Geneticist Lap-Chee Tsui and his team identified the ''CFTR'' gene in 1989 as the gene linked with CF (cystic fibrosis). The ''CFTR'' gene codes for an ABC transporter-class ion channel protein that conducts chloride and bicarbonate ions across epithelial cell membranes. Mutations of the ''CFTR'' gene affecting anion channel function lead to dysregulation of epithelial lining fluid (mucus) transport in the lung, pancreas and other organs, resulting in cystic fibrosis. Complications include thickened mucus in the lungs with frequent respiratory infections, and pancreatic insufficiency giving rise to malnutrition and diabetes. These conditions lead to chronic disability and reduced life expectancy. In male patients, the progressive obstruction and destruction of the developing vas deferens (spermatic cord) and epididymis appe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |