|
Iohexol
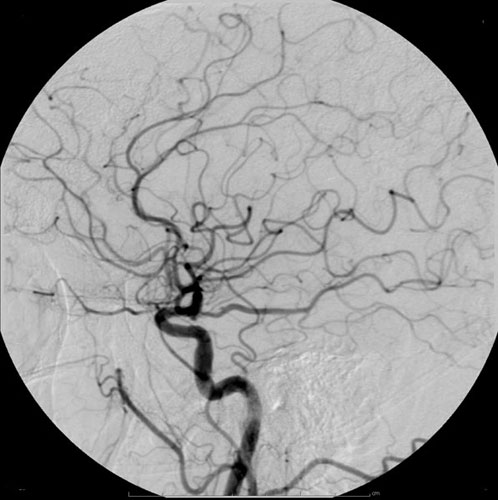
Iohexol, sold under the trade name Iodaque among others, is a contrast agent used for X-ray imaging. This includes when visualizing arteries, veins, ventricles of the brain, the urinary system, and joints, as well as during computed tomography (CT scan). It is given by mouth, injection into a vein, or into a body cavity. Side effects include vomiting, skin flushing, headache, itchiness, kidney problems, and low blood pressure. Less commonly allergic reactions or seizures may occur. Allergies to povidone-iodine or shellfish do not affect the risk of side effects more than other allergies. Use in the later part of pregnancy may cause hypothyroidism in the baby. Iohexol is an iodinated non-ionic radiocontrast agent. It is in the low osmolar family. Iohexol was approved for medical use in 1985. It is on the World Health Organization's List of Essential Medicines. Chemistry The osmolality of iohexol ranges from 322 mOsm/kg—approximately 1.1 times that of blood plasma� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiocontrast
Radiocontrast agents are substances used to enhance the visibility of internal structures in X-ray-based imaging techniques such as computed tomography (contrast CT), projectional radiography, and fluoroscopy. Radiocontrast agents are typically iodine, or more rarely barium sulfate. The contrast agents absorb external X-rays, resulting in decreased exposure on the X-ray detector. This is different from radiopharmaceuticals used in nuclear medicine which emit radiation. Magnetic resonance imaging (MRI) functions through different principles and thus MRI contrast agents have a different mode of action. These compounds work by altering the magnetic properties of nearby hydrogen nuclei. Types and uses Radiocontrast agents used in X-ray examinations can be grouped in positive (iodinated agents, barium sulfate), and negative agents (air, carbon dioxide, methylcellulose). Iodine (circulatory system) Iodinated contrast contains iodine. It is the main type of radiocontrast used for intra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intrathecal
Intrathecal administration is a route of administration for drugs via an injection into the spinal canal, or into the subarachnoid space (sin. ''intrathecal space'') so that it reaches the cerebrospinal fluid (CSF). It is useful in several applications, such as for spinal anesthesia, chemotherapy, or pain management. This route is also used to introduce drugs that fight certain infections, particularly post-neurosurgical. Typically, the drug is given this way to avoid being stopped by the blood–brain barrier, as it may not be able to pass into the brain when given orally. Drugs given by the intrathecal route often have to be compounded specially by a pharmacist or technician because they cannot contain any preservative or other potentially harmful inactive ingredients that are sometimes found in standard injectable drug preparations. Intrathecal pseudodelivery is a technique where the drug is encapsulated in a porous capsule that is placed in communication with the CSF. In this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low Blood Pressure
Hypotension, also known as low blood pressure, is a cardiovascular condition characterized by abnormally reduced blood pressure. Blood pressure is the force of blood pushing against the walls of the arteries as the heart pumps out blood and is indicated by two numbers, the systolic blood pressure (the top number) and the diastolic blood pressure (the bottom number), which are the maximum and minimum blood pressures within the cardiac cycle, respectively. A systolic blood pressure of less than 90 millimeters of mercury (mmHg) or diastolic of less than 60 mmHg is generally considered to be hypotension. Different numbers apply to children. However, in practice, blood pressure is considered too low only if noticeable symptoms are present. Symptoms may include dizziness, lightheadedness, confusion, feeling tired, weakness, headache, blurred vision, nausea, neck or back pain, an irregular heartbeat or feeling that the heart is skipping beats or fluttering, sweating, and fain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Library Of Medicine
The United States National Library of Medicine (NLM), operated by the United States federal government, is the world's largest medical library. Located in Bethesda, Maryland, the NLM is an institute within the National Institutes of Health. Its collections include more than seven million books, journals, technical reports, manuscripts, microfilms, photographs, and images on medicine and related sciences, including some of the world's oldest and rarest works. the acting director of the NLM was Stephen Sherry. History The precursor of the National Library of Medicine, established in 1836, was the Library of the Surgeon General's Office, a part of the office of the Surgeon General of the United States Army. The Armed Forces Institute of Pathology and its Medical Museum were founded in 1862 as the Army Medical Museum. Throughout their history the Library of the Surgeon General's Office and the Army Medical Museum often shared quarters. From 1866 to 1887, they were ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Plasma
Blood plasma is a light Amber (color), amber-colored liquid component of blood in which blood cells are absent, but which contains Blood protein, proteins and other constituents of whole blood in Suspension (chemistry), suspension. It makes up about 55% of the body's total blood volume. It is the Intravascular compartment, intravascular part of extracellular fluid (all body fluid outside cells). It is mostly water (up to 95% by volume), and contains important dissolved proteins (6–8%; e.g., serum albumins, globulins, and fibrinogen), glucose, clotting factors, electrolytes (, , , , , etc.), hormones, carbon dioxide (plasma being the main medium for excretory product transportation), and oxygen. It plays a vital role in an intravascular osmotic effect that keeps electrolyte concentration balanced and protects the body from infection and other blood-related disorders. Blood plasma can be separated from whole blood through blood fractionation, by adding an anticoagulant to a tube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmole (unit)
Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L). The osmolarity of a solution is usually expressed as Osm/L (pronounced "osmolar"), in the same way that the molarity of a solution is expressed as "M" (pronounced "molar"). Whereas molarity measures the number of moles of solute per unit volume of solution, osmolarity measures the number of particles on dissociation of osmotically active material (''osmoles of solute particles)'' per unit volume of solution. This value allows the measurement of the osmotic pressure of a solution and the determination of how the solvent will diffuse across a semipermeable membrane (osmosis) separating two solutions of different osmotic concentration. Unit The unit of osmotic concentration is the ''osmole''. This is a non- SI unit of measurement that defines the number of moles of solute that contribute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmolality
In chemistry, molality is a measure of the amount of solute in a solution relative to a given mass of solvent. This contrasts with the definition of ''molarity'' which is based on a given volume of solution. A commonly used unit for molality is the moles per kilogram (mol/kg). A solution of concentration 1 mol/kg is also sometimes denoted as 1 molal. The unit mol/kg requires that molar mass be expressed in kg/mol, instead of the usual g/mol or kg/kmol. Definition The molality (''b''), of a solution is defined as the amount of substance (in moles) of solute, ''n''solute, divided by the mass (in kg) of the solvent, ''m''solvent: :b = \frac. In the case of solutions with more than one solvent, molality can be defined for the mixed solvent considered as a pure pseudo-solvent. Instead of mole solute per kilogram solvent as in the binary case, units are defined as mole solute per kilogram mixed solvent. Origin The term ''molality'' is formed in analogy to ''molarity' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WHO Model List Of Essential Medicines
The WHO Model List of Essential Medicines (aka Essential Medicines List or EML), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health system. The list is frequently used by countries to help develop their own local lists of essential medicines. , more than 155 countries have created national lists of essential medicines based on the World Health Organization's model list. This includes both Developed country, developed and Developing country, developing countries. The list is divided into core items and complementary items. The core items are deemed to be the most cost-effective options for key health problems and are usable with little additional health care resources. The complementary items either require additional infrastructure such as specially trained health care providers or diagnostic equipment or have a lower cost–benefit ratio. About 25% of items are in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmolar
Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L). The osmolarity of a solution is usually expressed as Osm/L (pronounced "osmolar"), in the same way that the molarity of a solution is expressed as "M" (pronounced "molar"). Whereas molarity measures the number of moles of solute per unit volume of solution, osmolarity measures the number of particles on dissociation of osmotically active material (''osmoles of solute particles)'' per unit volume of solution. This value allows the measurement of the osmotic pressure of a solution and the determination of how the solvent will diffuse across a semipermeable membrane (osmosis) separating two solutions of different osmotic concentration. Unit The unit of osmotic concentration is the ''osmole''. This is a non- SI unit of measurement that defines the number of moles of solute that contribute t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
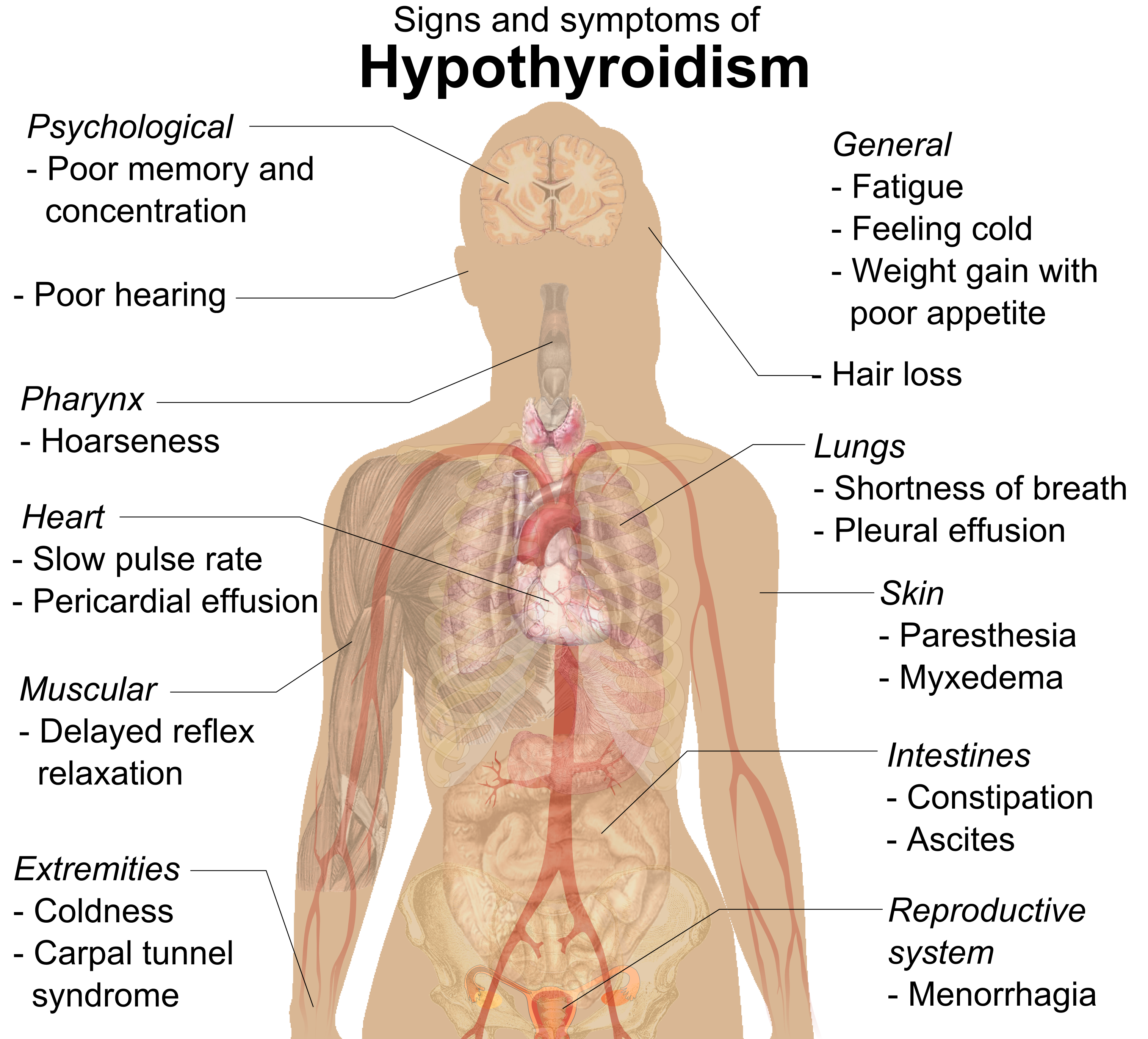
Hypothyroidism
Hypothyroidism is an endocrine disease in which the thyroid gland does not produce enough thyroid hormones. It can cause a number of symptoms, such as cold intolerance, poor ability to tolerate cold, fatigue, extreme fatigue, muscle aches, constipation, slow heart rate, Depression (mood), depression, and weight gain. Occasionally there may be swelling of the front part of the neck due to goiter. Untreated cases of hypothyroidism during pregnancy can lead to delays in child development, growth and intellectual development in the baby or congenital iodine deficiency syndrome. Worldwide, iodine deficiency, too little iodine in the diet is the most common cause of hypothyroidism. Hashimoto's thyroiditis, an autoimmune disease where the body's immune system reacts to the thyroid gland, is the most common cause of hypothyroidism in countries with sufficient dietary iodine. Less common causes include previous treatment with iodine-131, radioactive iodine, injury to the hypothalamus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pregnancy
Pregnancy is the time during which one or more offspring gestation, gestates inside a woman's uterus. A multiple birth, multiple pregnancy involves more than one offspring, such as with twins. Conception (biology), Conception usually occurs following sexual intercourse, vaginal intercourse, but can also occur through assisted reproductive technology procedures. A pregnancy may end in a Live birth (human), live birth, a miscarriage, an Abortion#Induced, induced abortion, or a stillbirth. Childbirth typically occurs around 40 weeks from the start of the Menstruation#Onset and frequency, last menstrual period (LMP), a span known as the Gestational age (obstetrics), ''gestational age''; this is just over nine months. Counting by Human fertilization#Fertilization age, ''fertilization age'', the length is about 38 weeks. Implantation (embryology), Implantation occurs on average 8–9 days after Human fertilization, fertilization. An ''embryo'' is the term for the deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |