|
Glucuronidation
Glucuronidation is often involved in drug metabolism of substances such as drugs, pollutants, bilirubin, androgens, estrogens, mineralocorticoids, glucocorticoids, fatty acid derivatives, retinoids, and bile acids. These linkages involve glycosidic bonds. Mechanism Glucuronidation consists of transfer of the glucuronic acid component of uridine diphosphate glucuronic acid to a substrate by any of several types of UDP-glucuronosyltransferase. UDP-glucuronic acid (glucuronic acid linked via a glycosidic bond to uridine diphosphate) is an intermediate in the process and is formed in the liver. One example is the N-glucuronidation of an aromatic amine, 4-aminobiphenyl, by UGT1A4 or UGT1A9 from human, rat, or mouse liver. : The substances resulting from glucuronidation are known as glucuronides (or glucuronosides) and are typically much more water- soluble than the non-glucuronic acid-containing substances from which they were originally synthesised. The human body uses g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucuronic Acid
Glucuronic acid (GCA, from ) is a uronic acid that was first isolated from urine (hence the name "uronic acid"). It is found in many natural gum, gums such as gum arabic ( 18%), xanthan, and kombucha tea and is important for the metabolism of microorganisms, plants and animals. Properties Glucuronic acid is a sugar acid derived from glucose, with its sixth carbon atom oxidized to a carboxylic acid. In living beings, this primary oxidation occurs with Uridine diphosphate glucose, UDP-α-D-glucose (UDPG), not with the free sugar. Glucuronic acid, like its precursor glucose, can exist as a linear (carboxo-)aldohexose ( 60,000 are too large for renal excretion and will be excreted with bile into the intestine. Neonates are deficient in this conjugating system, making them particularly vulnerable to drugs such as chloramphenicol, which is inactivated by the addition of glucuronic acid, resulting in gray baby syndrome. Bilirubin is excreted in the bile as bilirubin diglucuronid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UDP-glucuronyltransferase
Uridine 5'-diphospho-glucuronosyltransferase ( UDP-glucuronosyltransferase, UDPGT or UGT) is a microsomal glycosyltransferase () that catalyzes the transfer of the glucuronic acid component of UDP-glucuronic acid to a small hydrophobic molecule. This is a glucuronidation reaction. ''Alternative names:'' * glucuronyltransferase * UDP-glucuronyl transferase * UDP-GT Function Glucuronosyltransferases are responsible for the process of glucuronidation, a major part of phase II metabolism. Arguably the most important of the Phase II (conjugative) enzymes, UGTs have been the subject of increasing scientific inquiry since the mid-to-late 1990s. The reaction catalyzed by the UGT enzyme involves the addition of a glucuronic acid moiety to xenobiotics and is the most important pathway for the human body's elimination of the most frequently prescribed drugs. It is also the major pathway for foreign chemical (dietary, environmental, pharmaceutical) removal for most drugs, dietary subs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
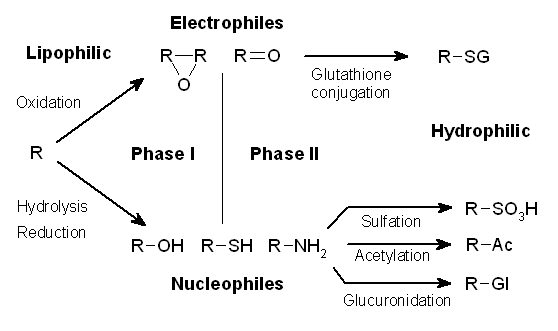
Drug Metabolism
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is the object of pharmacokinetics. Metabolism is one of the stages (see ADME) of the drug's transit through the body that involves the breakdown of the drug so that it can be excreted by the body. The metabolism of pharmaceutical drugs is an important as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Aminobiphenyl
4-Aminobiphenyl (4-ABP) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored. 4-Aminobiphenyl was commonly used in the past as a rubber antioxidant and an intermediate for dyes. Exposure to this aryl-amine can happen through contact with chemical dyes and from inhalation of cigarette smoke. Researches showed that 4-aminobiphenyl is responsible for bladder cancer in humans and dogs by damaging DNA. Due to its carcinogenic effects, commercial production of 4-aminobiphenyl ceased in the United States in the 1950s. Synthesis and reactivity Like other aniline derivatives, 4-aminobiphenyl is weakly Base (chemistry), basic. It is prepared by reduction of 4-Nitrobiphenyl, 4-nitrobiphenyl: : Together with other isomers, 4-nitrobiphenyl is obtained by nitration of biphenyl. 4-Aminobiphenyl can in principle be obtained by reduction of 4-azidobiphenyl with diphosphorus tetraiodide (P2I4). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucuronide
A glucuronide, also known as glucuronoside, is any substance produced by linking glucuronic acid to another substance via a glycosidic bond. The glucuronides belong to the glycosides. Glucuronidation, the conversion of chemical compounds to glucuronides, is a method that animals use to assist in the excretion of toxic substances, drugs or other substances that cannot be used as an energy source. Glucuronic acid is attached via a glycosidic bond to the substance, and the resulting glucuronide, which has a much higher water solubility than the original substance, is eventually excreted by the kidneys. Enzymes that cleave the glycosidic bond of a glucuronide are called glucuronidases. Classes of glucuronides Acyl glucuronide Carboxylic acids are a common functional group in many medications, such as NSAIDS, anticonvulsants, and diuretics. One common pathway for the clearance of carboxylic-acid-containing drugs is via glucuronidation. By conjugating one such drug to a glucur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase II Metabolism
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is the object of pharmacokinetics. Metabolism is one of the stages (see ADME) of the drug's transit through the body that involves the breakdown of the drug so that it can be excreted by the body. The metabolism of pharmaceutical drugs is an important aspec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bilirubin
Bilirubin (BR) (adopted from German, originally bili—bile—plus ruber—red—from Latin) is a red-orange compound that occurs in the normcomponent of the straw-yellow color in urine. Another breakdown product, stercobilin, causes the brown color of feces. Although bilirubin is usually found in animals rather than plants, at least one plant species, '' Strelitzia nicolai'', is known to contain the pigment. Structure Bilirubin consists of an open-chain tetrapyrrole. It is formed by oxidative cleavage of a porphyrin in heme, which affords biliverdin. Biliverdin is reduced to bilirubin. After conjugation with glucuronic acid, bilirubin is water-soluble and can be excreted. Bilirubin is structurally similar to the pigment phycobilin used by certain algae to capture light energy, and to the pigment phytochrome used by plants to sense light. All of these contain an open chain of four pyrrolic rings. Like these other pigments, some of the double-bonds in bilirubin isomer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen
Estrogen (also spelled oestrogen in British English; see spelling differences) is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal activity: estrone (E1), estradiol (E2), and estriol (E3). Estradiol, an estrane, is the most potent and prevalent. Another estrogen called estetrol (E4) is produced only during pregnancy. Estrogens are synthesized in all vertebrates and some insects. Quantitatively, estrogens circulate at lower levels than androgens in both men and women. While estrogen levels are significantly lower in males than in females, estrogens nevertheless have important physiological roles in males. Like all steroid hormones, estrogens readily diffuse across the cell membrane. Once inside the cell, they bind to and activate estrogen receptors (ERs) which in turn modulate the expression of many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liver
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of various proteins and various other Biochemistry, biochemicals necessary for digestion and growth. In humans, it is located in the quadrants and regions of abdomen, right upper quadrant of the abdomen, below the thoracic diaphragm, diaphragm and mostly shielded by the lower right rib cage. Its other metabolic roles include carbohydrate metabolism, the production of a number of hormones, conversion and storage of nutrients such as glucose and glycogen, and the decomposition of red blood cells. Anatomical and medical terminology often use the prefix List of medical roots, suffixes and prefixes#H, ''hepat-'' from ἡπατο-, from the Greek language, Greek word for liver, such as hepatology, and hepatitis The liver is also an accessory digestive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosidic Bond
A glycosidic bond or glycosidic linkage is a type of ether bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate. A glycosidic bond is formed between the hemiacetal or hemiketal group of a saccharide (or a molecule derived from a saccharide) and the hydroxyl group of some compound such as an alcohol. A substance containing a glycosidic bond is a glycoside. The term 'glycoside' is now extended to also cover compounds with bonds formed between hemiacetal (or hemiketal) groups of sugars and several chemical groups other than hydroxyls, such as -SR (thioglycosides), -SeR (selenoglycosides), -NR1R2 (N-glycosides), or even -CR1R2R3 (C-glycosides). Particularly in naturally occurring glycosides, the compound ROH from which the carbohydrate residue has been removed is often termed the aglycone, and the carbohydrate residue itself is sometimes referred to as the 'glycone'. S-, N-, C-, and O-glycosidic bonds Glycosidic bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hormone
A hormone (from the Ancient Greek, Greek participle , "setting in motion") is a class of cell signaling, signaling molecules in multicellular organisms that are sent to distant organs or tissues by complex biological processes to regulate physiology and behavior. Hormones are required for the normal development of animals, plants and fungi. Due to the broad definition of a hormone (as a signaling molecule that exerts its effects far from its site of production), numerous kinds of molecules can be classified as hormones. Among the substances that can be considered hormones, are eicosanoids (e.g. prostaglandins and thromboxanes), steroids (e.g. Estrogen, oestrogen and brassinosteroid), amino acid derivatives (e.g. epinephrine and auxin), protein or peptides (e.g. insulin and CLE peptides), and gases (e.g. ethylene and nitric oxide). Hormones are used to communicate between organ (anatomy), organs and Tissue (biology), tissues. In vertebrates, hormones are responsible for regulating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |