|
Fluorescence Intermittency
Fluorescence intermittency, or blinking, is the phenomenon of random switching between ON (bright) and OFF (dark) states of the emitter under its continuous excitation. It is a common property of the nanoscale emitters (molecular fluorophores, colloidal quantum dots) related to the competition between the radiative and non-radiative relaxation pathways. The peculiar feature of such blinking in most cases is the power-law (in contrast to exponential) statistics of the ON and OFF time distributions, meaning that the measurements of the time-averaged intensity of a single emitter is not reproducible in different experiments and implying a complex dynamics of the involved process. In other words, in one experiment the emitter can blink frequently, while in another it may stay ON (or OFF) for almost entire length of the experiment (even for extremely long measurement times). For CdSe-ZnS core-shell nanocrystals, "charge trapping" is the dominant theory explaining observed power-law blin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorophores
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalently bonded to a macromolecule, serving as a marker (or dye, or tag, or reporter) for affine or bioactive reagents (antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, i.e., fluorescent imaging and spectroscopy. Fluorescein, via its amine-reactive isothiocyanate derivative fluorescein isothiocyanate (FITC), has been one of the most popular fluorophores. From a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
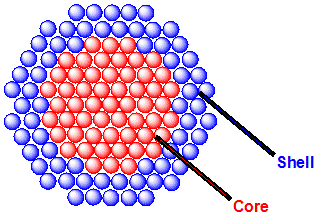
Quantum Dots
Quantum dots (QDs) are semiconductor particles a few nanometres in size, having optical and electronic properties that differ from those of larger particles as a result of quantum mechanics. They are a central topic in nanotechnology. When the quantum dots are illuminated by UV light, an electron in the quantum dot can be excited to a state of higher energy. In the case of a semiconducting quantum dot, this process corresponds to the transition of an electron from the valence band to the conductance band. The excited electron can drop back into the valence band releasing its energy as light. This light emission (photoluminescence) is illustrated in the figure on the right. The color of that light depends on the energy difference between the conductance band and the valence band, or the transition between discrete energy states when band structure is no longer a good definition in QDs. In the language of materials science, nanoscale semiconductor materials tightly confine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Power-law
In statistics, a power law is a functional relationship between two quantities, where a relative change in one quantity results in a proportional relative change in the other quantity, independent of the initial size of those quantities: one quantity varies as a power of another. For instance, considering the area of a square in terms of the length of its side, if the length is doubled, the area is multiplied by a factor of four. Empirical examples The distributions of a wide variety of physical, biological, and man-made phenomena approximately follow a power law over a wide range of magnitudes: these include the sizes of craters on the moon and of solar flares, the foraging pattern of various species, the sizes of activity patterns of neuronal populations, the frequencies of words in most languages, frequencies of family names, the species richness in clades of organisms, the sizes of power outages, volcanic eruptions, human judgments of stimulus intensity and many other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
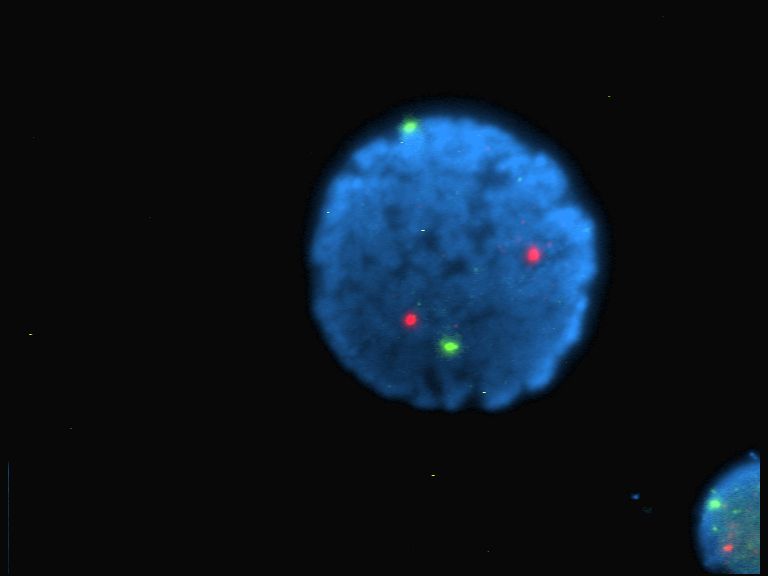
Fluorescence Intermittency In Colloidal Nanocrystals
Blinking colloidal nanocrystals is a phenomenon observed during studies of single colloidal nanocrystals that show that they randomly turn their photoluminescence on and off even under continuous light illumination. This has also been described as luminescence intermittency. Similar behavior has been observed in crystals made of other materials. For example, porous silicon also exhibits this affect. Colloidal nanocrystals Colloidal nanocrystals are a new class of optical materials that essentially constitute a new form of matter that can be considered as "artificial atoms." Like atoms, they have discrete optical energy spectra that are tunable over a wide range of wavelengths. The desired behavior and transmission directly correlates to their size. To change the emitted wavelength, the crystal is grown larger or smaller. Their electronic and optical properties can be controlled by this method. For example, to change the emission from one visible wavelength to another simply us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |