|
Epoxydocosapentaenoic Acid
Epoxide docosapentaenoic acids (epoxydocosapentaenoic acids, EDPs, or EpDPEs) are metabolites of the 22-carbon straight-chain omega-3 fatty acid, docosahexaenoic acid (DHA). Cell types that express certain cytochrome P450 (CYP) epoxygenases metabolize polyunsaturated fatty acid's (PUFAs) by converting one of their double bonds to an epoxide. In the best known of these metabolic pathways, cellular CYP epoxygenases metabolize the 20-carbon straight-chain omega-6 fatty acid, arachidonic acid, to epoxyeicosatrienoic acids (EETs); another CYP epoxygenase pathway metabolizes the 20-carbon omega-3 fatty acid, eicosapentaenoic acid (EPA), to epoxyeicosatetraenoic acids (EEQs). CYP epoxygenases similarly convert various other PUFAs to epoxides (see epoxygenase) These epoxide metabolites have a variety of activities. However, essentially all of them are rapidly converted to their corresponding, but in general far less active, Vicinal (chemistry) dihydroxy fatty acids by ubiquitous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomers
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other. A molecule with chirality rotates plane-polarized light. A mixture of equals am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP4F8
Cytochrome P450 4F8 is a protein that in humans is encoded by the ''CYP4F8'' gene. Function This gene, CYP4F8, encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. This protein localizes to the endoplasmic reticulum and functions as a 19-hydroxylase of the arachidonic acid metabolite, prostaglandin H2 (PGH2) and the Dihomo-γ-linolenic acid metabolite PGH1 in seminal vesicles. This gene is part of a cluster of cytochrome P450 genes on chromosome 19. Another member of this family, CYP4F3, is approximately 18 kb away. In addition to seminal vesicles, CYP4F8 is expressed in kidney, prostate, epidermis, and corneal epithelium, and its mRNA has been found in retina; CYP4F8 is also greatly up-regulated in psoriatic skin. In addition to its ability to metabolize and presumably thereby to inactivate or reduce the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP4A11
Cytochrome P450 4A11 is a protein that in humans is codified by the ''CYP4A11'' gene. Function This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. This protein localizes to the endoplasmic reticulum and hydroxylates medium-chain fatty acids such as laurate and myristate. CYP4A11 is highly expressed in the liver and kidney. CYP4A11 along with CYP4A22, CYP4F2, and CYP4F3 metabolize arachidonic acid to 20-Hydroxyeicosatetraenoic acid (20-HETE) by an Omega oxidation reaction with the predominant 20-HETE-synthesizing enzymes in humans being CYP4F2 followed by CYP4A11; 20-HETE regulates blood flow, vascularization, blood pressure, and kidney tubule absorption of ions in rodents and possibly humans. Gene polymorphism variants of CYP4A11 are associated with the development of hypertension and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2E1
Cytochrome P450 2E1 (abbreviated CYP2E1, ) is a member of the cytochrome P450 mixed-function oxidase system, which is involved in the metabolism of xenobiotics in the body. This class of enzymes is divided up into a number of subcategories, including CYP1, CYP2, and CYP3, which as a group are largely responsible for the breakdown of foreign compounds in mammals. While CYP2E1 itself carries out a relatively low number of these reactions (~4% of known P450-mediated drug oxidations), it and related enzymes CYP1A2 and CYP3A4 are responsible for the breakdown of many toxic environmental chemicals and carcinogens that enter the body, in addition to basic metabolic reactions such as fatty acid oxidations. Function CYP2E1 is a membrane protein expressed in high levels in the liver, where it composes nearly 50% of the total hepatic cytochrome P450 mRNA and 7% of the hepatic cytochrome P450 protein. The liver is therefore where most drugs undergo deactivation by CYP2E1, either directly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2C18
Cytochrome P450 2C18 is a protein that in humans is encoded by the ''CYP2C18'' gene. Function This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. This protein localizes to the endoplasmic reticulum but its specific substrate has not yet been determined. The gene is located within a cluster of cytochrome P450 genes on chromosome 10q24. An additional gene, CYP2C17, was once thought to exist; however, CYP4217 is now considered an artefact based on a chimera of CYP2C18 and CYP2C19. CYP2C18 also possesses epoxygenase activity: it can attack various long-chain polyunsaturated fatty acids at their double (i.e. alkene) bonds to form epoxide products that act as signaling agents. It metabolizes: 1) arachidonic acid to various epoxyeicosatrienoic acids (also termed EETs); 2) linoleic acid to 9,10-epoxy oct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP1A2
Cytochrome P450 1A2 (abbreviated CYP1A2), a member of the cytochrome P450 mixed-function oxidase system, is involved in the metabolism of xenobiotics in the human body. In humans, the CYP1A2 enzyme is encoded by the ''CYP1A2'' gene. Function CYP1A2 is a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. CYP1A2 localizes to the endoplasmic reticulum and its expression is induced by some polycyclic aromatic hydrocarbons (PAHs), some of which are found in cigarette smoke. The enzyme's endogenous substrate is unknown; however, it is able to metabolize some PAHs to carcinogenic intermediates. Other xenobiotic substrates for this enzyme include caffeine, aflatoxin B1, and paracetamol (acetaminophen). The transcript from this gene contains four Alu sequences flanked by direct repeats in the 3' untranslated region. CYP1A2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP1A1
Cytochrome P450, family 1, subfamily A, polypeptide 1 is a protein that in humans is encoded by the ''CYP1A1'' gene. The protein is a member of the cytochrome P450 superfamily of enzymes. Function Metabolism of xenobiotics and drugs CYP1A1 is involved in phase I xenobiotic and drug metabolism (one substrate of it is theophylline). It is inhibited by hesperetin (a flavonoid found in lime, sweet orange), fluoroquinolones and macrolides and induced by aromatic hydrocarbons. CYP1A1 is also known as AHH (aryl hydrocarbon hydroxylase). It is involved in the metabolic activation of aromatic hydrocarbons ( polycyclic aromatic hydrocarbons, PAH), for example, benzo yrene (BaP), by transforming it to an epoxide. In this reaction, the oxidation of benzo yrene is catalysed by CYP1A1 to form BaP-7,8-epoxide, which can be further oxidized by epoxide hydrolase (EH) to form BaP-7,8-dihydrodiol. Finally, CYP1A1 catalyses this intermediate to form BaP-7,8-dihydrodiol-9,10-epoxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxyeicosatrienoic Acid
The epoxyeicosatrienoic acids or EETs are signaling molecules formed within various types of cells by the metabolism of arachidonic acid by a specific subset of Cytochrome P450 enzymes termed cytochrome P450 epoxygenases. These nonclassic eicosanoids are generally short-lived, being rapidly converted from epoxides to less active or inactive dihydroxy-eicosatrienoic acids (diHETrEs) by a widely distributed cellular enzyme, Soluble epoxide hydrolase (sEH), also termed Epoxide hydrolase 2. The EETs consequently function as transiently acting, short-range hormones; that is, they work locally to regulate the function of the cells that produce them (i.e. they are autocrine agents) or of nearby cells (i.e. they are paracrine agents). The EETs have been most studied in animal models where they show the ability to lower blood pressure possibly by a) stimulating arterial vasorelaxation and b) inhibiting the kidney's retention of salts and water to decrease intravascular blood volume. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
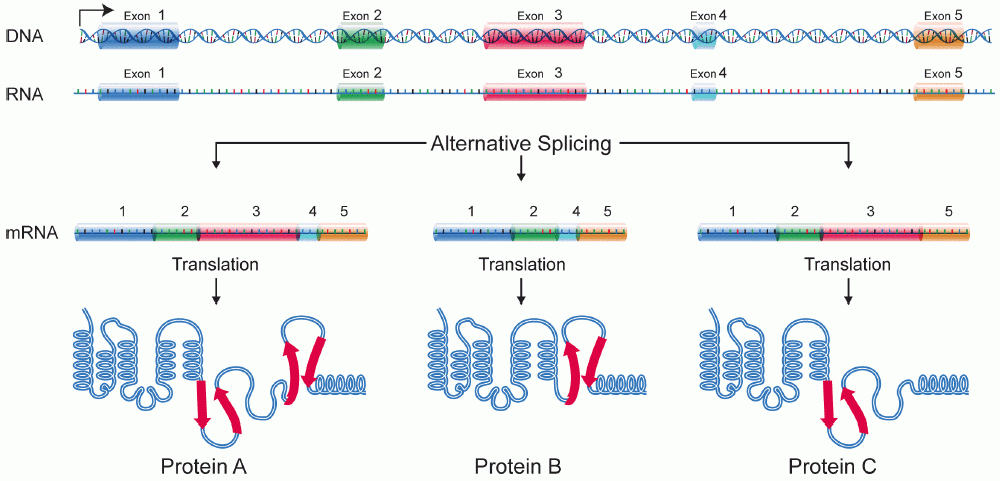
Isoforms
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have unique functions. A set of protein isoforms may be formed from alternative splicings, variable promoter usage, or other post-transcriptional modifications of a single gene; post-translational modifications are generally not considered. (For that, see Proteoforms.) Through RNA splicing mechanisms, mRNA has the ability to select different protein-coding segments ( exons) of a gene, or even different parts of exons from RNA to form different mRNA sequences. Each unique sequence produces a specific form of a protein. The discovery of isoforms could explain the discrepancy between the small number of protein coding regions genes revealed by the human genome project and the large diversity of proteins seen in an organism: differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2S1
Cytochrome P450 2S1 is a protein that in humans is encoded by the ''CYP2S1'' gene. The gene is located in chromosome 19q13.2 within a cluster including other CYP2 family members such as CYP2A6, CYP2A13, CYP2B6, and CYP2F1. Expression CYP2S1 is highly expressed in epithelial tissues of the respiratory, gastrointestinal, urinary tracts, and skin and in leukocytes of the monocyte/macrophage and lymphocyte series; it is also expressed throughout Embryogenesis and, as discussed below, certain types of cancers. Function This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. This protein localizes to the endoplasmic reticulum. In rodents, the homologous protein has been shown to metabolize certain carcinogens although its specific function(s) in humans has not been clearly determined. In in vitro studi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2J2
Cytochrome P450 2J2 (CYP2J2) is a protein that in humans is encoded by the ''CYP2J2'' gene. CYP2J2 is a member of the cytochrome P450 superfamily of enzymes. The enzymes are oxygenases which catalyze many reactions involved in the metabolism of drugs and other xenobiotics) as well as in the synthesis of cholesterol, steroids and other lipids. Protein structure The CYP2J2 contains the following domains: • Hydrophobic binding domains • F-G loop (containing non-conservative mutations) primary membrane binding motif The protein also contains an N-terminal anchor. F-G loop The F-G loop mediates the binding and passage of substrates, and its hydrophobic region containing residues Trp-235, Phe-239 and Ille-236 allows the enzyme to interact with cellular membranes. Mutations to hydrophilic residues in the F-G loop alter the binding mechanism by changing insertion depth of the enzyme into the membrane. Tissue distribution CYP2J2 is expressed predominately in the heart an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |