|
Electron Energy Loss Spectroscopy
Electron energy loss spectroscopy (EELS) is a form of electron microscopy in which a material is exposed to a beam of electrons with a known, narrow range of kinetic energies. Some of the electrons will undergo inelastic scattering, which means that they lose energy and have their paths slightly and randomly deflected. The amount of energy loss can be measured via an electron spectrometer and interpreted in terms of what caused the energy loss. Inelastic interactions include phonon excitations, inter- and intra- band transitions, plasmon excitations, inner shell ionizations, and Cherenkov radiation. The inner-shell ionizations are particularly useful for detecting the elemental components of a material. For example, one might find that a larger-than-expected number of electrons comes through the material with 285 eV less energy than they had when they entered the material. This is approximately the amount of energy needed to remove an inner-shell electron from a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Electron Energy Loss Spectrum Feature Overview
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up and down quarks. Electrons are extremely lightweight particles that orbit the positively charged nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electrons are the least tightly bound and are responsible for the formation of chemical bonds between atoms to create molecules and crystals. These valence electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Energy-dispersive X-ray Spectroscopy
Energy-dispersive X-ray spectroscopy (EDS, EDX, EDXS or XEDS), sometimes called energy dispersive X-ray analysis (EDXA or EDAX) or energy dispersive X-ray microanalysis (EDXMA), is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on an interaction of some source of X-ray excitation and a sample. Its characterization capabilities are due in large part to the fundamental principle that each element has a unique atomic structure allowing a unique set of peaks on its electromagnetic emission spectrum (which is the main principle of spectroscopy). The peak positions are predicted by the Moseley's law with accuracy much better than experimental resolution of a typical EDX instrument. To stimulate the emission of characteristic X-rays from a specimen a beam of electrons or X-ray is focused into the sample being studied. At rest, an atom within the sample contains ground state (or unexcited) electrons in discrete energy levels ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Diamond Anvil Cell
A diamond anvil cell (DAC) is a high-pressure device used in geology, engineering, and materials science experiments. It permits the compression of a small (sub- millimeter-sized) piece of material to extreme pressures, typically up to around 100–200 gigapascals, although it is possible to achieve pressures up to 770 gigapascals (7,700,000 bars or 7.7 million atmospheres). The device has been used to recreate the pressure existing deep inside planets to synthesize materials and phases not observed under normal ambient conditions. Notable examples include the non-molecular ice X, polymeric nitrogen and metallic phases of xenon, lonsdaleite, and potentially metallic hydrogen. A DAC consists of two opposing diamonds with a sample compressed between the polished culets (tips). Pressure may be monitored using a reference material whose behavior under pressure is known. Common pressure standards include ruby fluorescence, and various structurally simp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Scanning Transmission Electron Microscopy
A scanning transmission electron microscope (STEM) is a type of transmission electron microscope (TEM). Pronunciation is [stɛm] or [ɛsti:i:ɛm]. As with a conventional transmission electron microscope (CTEM), images are formed by electrons passing through a sufficiently thin specimen. However, unlike CTEM, in STEM the electron beam is focused to a fine spot (with the typical spot size 0.05 – 0.2 nm) which is then scanned over the sample in a raster illumination system constructed so that the sample is illuminated at each point with the beam parallel to the optical axis. The rastering of the beam across the sample makes STEM suitable for analytical techniques such as Z-contrast annular dark-field imaging, and spectroscopic mapping by energy-dispersive X-ray spectroscopy, energy dispersive X-ray (EDX) spectroscopy, or electron energy loss spectroscopy (EELS). These signals can be obtained simultaneously, allowing direct correlation of images and spectroscopic data. A ty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Transmission Electron Microscopy
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a grid. An image is formed from the interaction of the electrons with the sample as the beam is transmitted through the specimen. The image is then magnified and focused onto an imaging device, such as a fluorescent screen, a layer of photographic film, or a detector such as a scintillator attached to a charge-coupled device or a direct electron detector. Transmission electron microscopes are capable of imaging at a significantly higher resolution than light microscopes, owing to the smaller de Broglie wavelength of electrons. This enables the instrument to capture fine detail—even as small as a single column of atoms, which is thousands of times smaller than a resolvable object seen in a light microscope. Transmission electron micr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Detectors For Transmission Electron Microscopy
There are a variety of technologies available for detecting and recording the images, diffraction patterns, and electron energy loss spectra produced using transmission electron microscopy (TEM). Traditional detection techniques Traditionally, TEM images or diffraction patterns could be observed using a fluorescent viewing screen, consisting of powdered ZnS or ZnS/CdS, which is excited by the electron beam via cathodoluminescence. Once the microscopist could see a suitable image on their viewing screen, images could then be recorded using photographic film. For electron microscopes, film typically consisted of a gelatin and silver halide emulsion layer on a plastic support base. The silver halide would be converted to silver upon exposure to the electron beam, and the film could then be chemically developed to form an image, which could be digitized for analysis using a film scanner. In modern TEMs, film has largely been replaced by electronic detectors. CCD cameras Charge co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
White Noise
In signal processing, white noise is a random signal having equal intensity at different frequencies, giving it a constant power spectral density. The term is used with this or similar meanings in many scientific and technical disciplines, including physics, acoustical engineering, telecommunications, and statistical forecasting. White noise refers to a statistical model for signals and signal sources, not to any specific signal. White noise draws its name from white light, although light that appears white generally does not have a flat power spectral density over the visible band. In discrete time, white noise is a discrete signal whose samples are regarded as a sequence of serially uncorrelated random variables with zero mean and finite variance; a single realization of white noise is a random shock. In some contexts, it is also required that the samples be independent and have identical probability distribution (in other words independent and identically distribu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Shot Noise
Shot noise or Poisson noise is a type of noise which can be modeled by a Poisson process. In electronics shot noise originates from the discrete nature of electric charge. Shot noise also occurs in photon counting in optical devices, where shot noise is associated with the particle nature of light. Origin In a statistical experiment such as tossing a fair coin and counting the occurrences of heads and tails, the numbers of heads and tails after many throws will differ by only a tiny percentage, while after only a few throws outcomes with a significant excess of heads over tails or vice versa are common; if an experiment with a few throws is repeated over and over, the outcomes will fluctuate a lot. From the law of large numbers, one can show that the relative fluctuations reduce as the reciprocal square root of the number of throws, a result valid for all statistical fluctuations, including shot noise. Shot noise exists because phenomena such as light and electric curren ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Vibrational Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
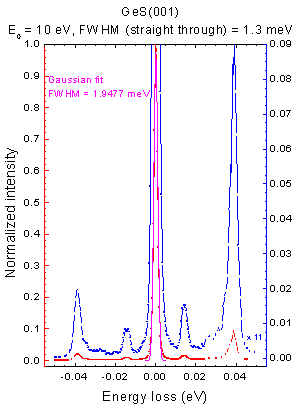
High Resolution Electron Energy Loss Spectroscopy
High resolution electron energy loss spectroscopy (HREELS) is a tool used in surface science. The inelastic scattering of electrons from surfaces is utilized to study electronic excitations or vibrational modes of the surface of a material or of molecules adsorbed to a surface. In contrast to other electron energy loss spectroscopies (EELS), HREELS deals with small energy losses in the range of 10−3 eV to 1 eV. It plays an important role in the investigation of surface structure, catalysis, dispersion of surface phonons and the monitoring of epitaxial growth. Overview of HREELS In general, electron energy loss spectroscopy is based on the energy losses of electrons when inelastically scattered on matter. An incident beam of electrons with a known energy (Ei) is scattered on a sample. The scattering of these electrons can excite the electronic structure of the sample. If this is the case the scattered electron loses the specific energy (ΔE) needed to cause the excitation. Those s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
X-ray Absorption Spectroscopy
X-ray absorption spectroscopy (XAS) is a set of advanced techniques used for probing the local environment of matter at atomic level and its electronic structure. The experiments require access to synchrotron radiation facilities for their intense and tunable X-ray beams. Samples can be in the gas phase, solutions, or solids. Background XAS data are obtained by tuning the photon energy, using a crystalline monochromator, to a range where core electrons can be excited (0.1-100 keV). The edges are, in part, named by which core electron is excited: the principal quantum numbers n = 1, 2, and 3, correspond to the K-, L-, and M-edges, respectively. For instance, excitation of a 1s electron occurs at the metal K-edge, K-edge, while excitation of a 2s or 2p electron occurs at an metal L-edge, L-edge (Figure 1). There are three main regions found on a spectrum generated by XAS data, which are then thought of as separate spectroscopic techniques (Figure 2): # The ''absorption thresho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Electron Energy Loss Spectroscopy Coreloss Lsmo
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up and down quarks. Electrons are extremely lightweight particles that orbit the positively charged nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electrons are the least tightly bound and are responsible for the formation of chemical bonds between atoms to create molecules and crystals. These valence electrons also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |