|
Electroelution
Electroelution is a method used to extract a nucleic acid or a protein sample from an electrophoresis gel by applying a negative Electric current, current in the plane of the smallest dimension of the gel, drawing the macromolecule to the surface for extraction and subsequent analysis. For example, electroblotting is based upon the same principle. DNA extraction Using this method, DNA fragments can be recovered from a particular region of agarose or polyacrylamide gels. The gel piece containing the fragment is excised (cut out from the whole gel) and placed in a Dialysis (chemistry), dialysis bag with Buffer solution, buffer. Electrophoresis causes the DNA to migrate out of the gel into the dialysis bag buffer. The DNA fragments are recovered from this buffer and purified, using phenol–chloroform extraction followed by ethanol precipitation. This method is simple, rapid and yields high recovery (75%) of DNA fragments from gel pieces. Protein extraction QPNC-PAGE, Preparative nati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
QPNC-PAGE
QPNC-PAGE, or Quantitative Preparative Native Continuous Polyacrylamide Gel Electrophoresis, is a bioanalytical, high-resolution and high-precision zone electrophoresis technique applied in biochemistry and bioinorganic chemistry to separate proteins or protein isoforms by isoelectric point and by continuous elution from a gel column for further characterization. This standardized 1-D hybrid variant of native gel electrophoresis and preparative polyacrylamide gel electrophoresis is used to quantitatively resolve physiological concentrations of macromolecules with high recovery, for example, into active or native metalloproteins in biological samples or into properly and improperly folded metal cofactor-containing proteins in complex protein mixtures. Introduction Proteins perform several functions in living organisms, including catalytic reactions and transport of molecules or ions within the cells, the organs or the whole body. The understanding of the processes in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
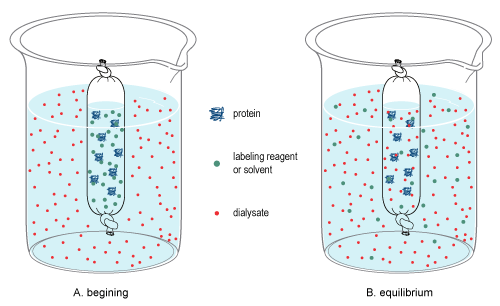
Dialysis (chemistry)
In chemistry, dialysis is the process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing. Dialysis is a common laboratory technique that operates on the same principle as medical dialysis. In the context of life science research, the most common application of dialysis is for the removal of unwanted small molecules such as salts, reducing agents, or dyes from larger macromolecules such as proteins, DNA, or polysaccharides. Dialysis is also commonly used for buffer exchange and drug binding studies. The concept of dialysis was introduced in 1861 by the Scottish chemist Thomas Graham. He used this technique to separate sucrose (small molecule) and gum Arabic solutes (large molecule) in aqueous solution. He called the diffusible solutes crystalloids and those that would not pass the membrane colloids. From this concept dialysis can be defined as a spontaneous separation process of suspended ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Elution
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent: washing of loaded ion-exchange resins to remove captured ions, or eluting proteins or other biopolymers from an electrophoresis or chromatography column. In a liquid chromatography experiment, for example, an analyte is generally adsorbed by ("bound to") an adsorbent in a liquid chromatography column. The adsorbent, a solid phase, called a "stationary phase", is a powder which is coated onto a solid support. Based on an adsorbent's composition, it can have varying affinities to "hold onto" other molecules—forming a thin film on the surface of its particles. Elution then is the process of removing analytes from the adsorbent by running a solvent, called an eluent, past the adsorbent–analyte complex. As the solvent molecules "elute", or travel down through the chromatography column, they can either pass by the adsorbent–analyte complex or displac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins. Abundance It is estimated that approximately half of all proteins contain a metal. In another estimate, about one quarter to one third of all proteins are proposed to require metals to carry out their functions. Thus, metalloproteins have many different functions in cells, such as storage and transport of proteins, enzymes and signal transduction proteins, or infectious diseases. The abundance of metal binding proteins may be inherent to the amino acids that proteins use, as even artificial proteins without evolutionary history will readily bind metals. Most metals in the human body are bound to proteins. For instance, the relatively high concentration of iron in the human body ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ethanol Precipitation
Ethanol precipitation is a method used to purify and/or concentrate RNA, DNA, and polysaccharides such as pectin and xyloglucan from aqueous solutions by adding salt and ethanol as an antisolvent. In DNA extraction, after separating DNA from other cell constituents in water, DNA is precipitated out of solution by neutralizing it with positively charged ions. The addition of ethanol to the solution is necessary to reduce the polarity of the solvent and allow the positively charged ions to interact with the negatively charged phosphate groups of DNA. DNA precipitation Theory DNA is typically separated from other cell constituents in a two-phase solution of phenol and water. Due to its highly charged phosphate backbone DNA is polar and will concentrate in the water phase while lipids and proteins will concentrate in the phenol phase. To precipitate the DNA out of the water, the negatively charged phosphate groups of the DNA backbone are neutralized by the addition of positively ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phenol–chloroform Extraction
Phenol–chloroform extraction is a liquid-liquid extraction technique in molecular biology used to separate nucleic acids from proteins and lipids. Process Aqueous samples, lysed cells, or homogenised tissue are mixed with equal volumes of a phenol:chloroform mixture. This mixture is then centrifuged. Because the phenol:chloroform mixture is immiscible with water, the centrifuge will cause two distinct phases to form: an upper aqueous phase, and a lower organic phase. The aqueous phase rises to the top because it is less dense than the organic phase containing the phenol:chloroform. This difference in density is why phenol, which only has a slightly higher density than water, must be mixed with chloroform to form a mixture with a much higher density than water. The hydrophobic lipids will partition into the lower organic phase, and the proteins will remain at the interphase between the two phases, while the nucleic acids (as well as other contaminants such as salts, sugars, etc.) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Buffer Solution
A buffer solution is a solution where the pH does not change significantly on dilution or if an acid or base is added at constant temperature. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen ion concentration increas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Polyacrylamide
Polyacrylamide (abbreviated as PAM or pAAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, mainly for water treatment and the paper and mineral industries. Physicochemical properties Polyacrylamide is a polyolefin. It can be viewed as polyethylene with amide substituents on alternating carbons. Unlike various nylons, polyacrylamide is not a polyamide because the amide groups are not in the polymer backbone. Owing to the presence of the amide (CONH2) groups, alternating carbon atoms in the backbone are stereogenic (colloquially: chiral). For this reason, polyacrylamide exists in atactic, syndiotactic, and isotactic forms, although this aspect is rarely discussed. The polymerization is initiated with radicals and is assumed to be stereorandom. Copolymers and modified polymers Linear polyacrylamide is a water-soluble polymer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nucleic Acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). If the sugar is ribose, the polymer is RNA; if the sugar is deoxyribose, a variant of ribose, the polymer is DNA. Nucleic acids are chemical compounds that are found in nature. They carry information in cells and make up genetic material. These acids are very common in all living things, where they create, encode, and store information in every living cell of every outline of life forms, life-form on Earth. In turn, they send and express that information inside and outside the cell nucleus. From the inner workings of the cell to the young of a living thing, they contain and provide information via the nucleic acid sequence. This gives the RNA and DNA their unmistakable 'la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Agarose
Agarose is a heteropolysaccharide, generally extracted from certain red algae. It is a linear polymer made up of the repeating unit of agarobiose, which is a disaccharide made up of D-galactose and 3,6-anhydro-L-galactopyranose. Agarose is one of the two principal components of agar, and is purified from agar by removing agar's other component, agaropectin. Agarose is frequently used in molecular biology for the separation of large molecules, especially DNA, by electrophoresis. Slabs of agarose gels (usually 0.7 - 2%) for electrophoresis are readily prepared by pouring the warm, liquid solution into a mold. A wide range of different agaroses of varying molecular weights and properties are commercially available for this purpose. Agarose may also be formed into beads and used in a number of chromatographic methods for protein purification. Structure Agarose is a linear polymer with a molecular weight of about 120,000, consisting of alternating D- galactose and 3,6-an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Electroblotting
Electroblotting is a method in molecular biology/biochemistry/immunogenetics to transfer proteins or nucleic acids onto a membrane by using PVDF or nitrocellulose, after gel electrophoresis. The protein or nucleic acid can then be further analyzed using probes such as specific antibodies, ligands like lectins, or stains. This method can be used with all polyacrylamide and agarose gels. An alternative technique for transferring proteins from a gel is capillary blotting. Development This technique was patented in 1989 by William J. Littlehales under the title "Electroblotting technique for transferring specimens from a polyacrylamide electrophoresis or like gel onto a membrane. Electroblotting procedure This technique relies upon current and a transfer buffer solution to drive proteins or nucleic acids onto a membrane. Following electrophoresis, a standard tank or semi-dry blotting transfer system is set up. A stack is put together in the following order from cathode to anode: spon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |