|
Divergent Synthesis
In chemistry a divergent synthesis is a strategy with the aim to improve the efficiency of chemical synthesis. It is often an alternative to convergent synthesis or linear synthesis. In one strategy divergent synthesis aims to generate a library of chemical compounds by first reacting a molecule with a set of reactants. The next generation of compounds is generated by further reactions with each compound in generation 1. This methodology quickly diverges to large numbers of new compounds *A generates A1, A2, A3, A4, A5 in generation 1 *A1 generates A11, A12, A13 in generation 2 and so on. An entire library of new chemical compounds, for instance saccharides, can be screened for desirable properties. In another strategy divergent synthesis starts from a molecule as a central core from which successive generations of building blocks are added. A good example is the divergent synthesis of dendrimers, for example, where in each generation a new monomer reacts to the growing surface ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work ( pharmacology), and how to collec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hoveyda–Grubbs Catalyst
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis. First-generation catalyst In the 1960s, ruthenium trichloride was found to catalyze olefin metathesis. Processes were commercialized based on these discoveries. These ill-defined but highly active homogeneous catalysts remain in industrial use. The first well-defined ruthenium catalyst was reported in 1992. It was prepared from RuCl2(PP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
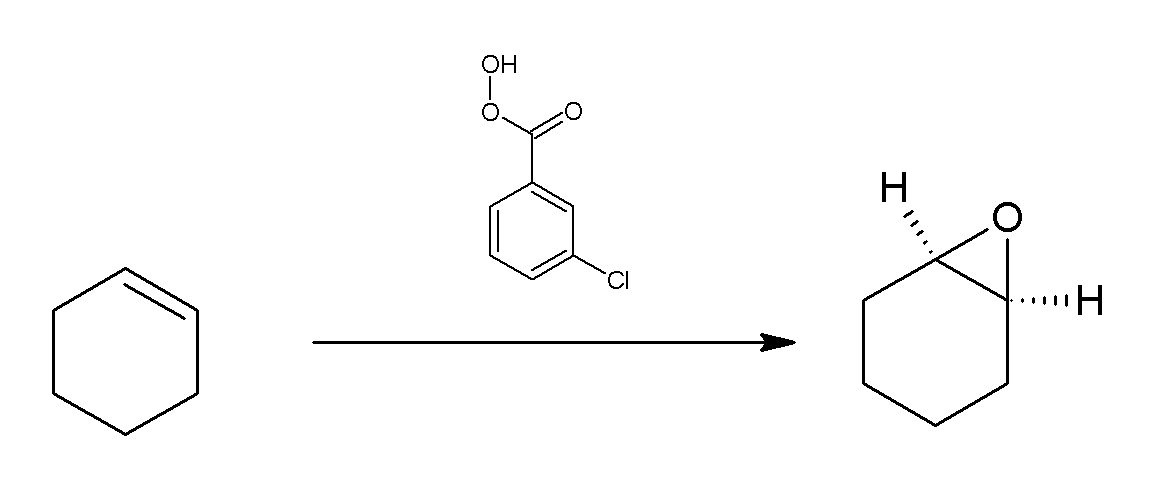
MCPBA
''meta''-Chloroperoxybenzoic acid (mCPBA or ''m''CPBA) is a peroxycarboxylic acid. A white solid, it is used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material. Preparation and purification mCPBA can be prepared by reacting m-Chlorobenzoyl chloride with a basic solution of hydrogen peroxide, followed by acidification. It is sold commercially as a shelf-stable mixture that is less than 72% mCPBA, with the balance made up of ''m''-chlorobenzoic acid (10%) and water. The peroxyacid can be purified by washing the commercial material with a sodium hydroxide and potassium phosphate solution buffered at pH = 7.5. Peroxyacids are generally slightly less acidic than their carboxylic acid counterparts, so one can extract the acid impurity by careful control of pH. The purified material is reasonably stable against d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Oxidation
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen, respectively.''Organic Redox Systems: Synthesis, Properties, and Applications'', Tohru Nishinaga 2016 Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation: When methane is oxidized to carbon dioxide its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include oxidation of alcohols to aldehydes. In oxidation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, methane, ammonia, and water. It is an ionic material that is insoluble in organic solvents (although soluble in molten Na), consistent with the fact that H− ions do not exist in solution. Because of the insolubility of NaH, all reactions involving NaH occur at the surface of the solid. Basic properties and structure NaH is produced by the direct reaction of hydrogen and liquid sodium.Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. . Pure NaH is colorless, although samples generally appear grey. NaH is ca. 40% denser than Na (0.968 g/cm3). NaH, like LiH, KH, RbH, and CsH, adopts the NaCl crystal structure. In this motif, each Na+ ion is surrounded by six H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esterification
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pauson–Khand Reaction
The Pauson–Khand reaction (or PKR or PK-type reaction) is a chemical reaction described as a 2+2+1.html" ;"title="/nowiki>2+2+1">/nowiki>2+2+1/nowiki> cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β- cyclopentenone. Ihsan Ullah Khand (1935-1980) discovered the reaction around 1970, while working as a postdoctoral associate with Peter Ludwig Pauson (1925–2013) at the University of Strathclyde in Glasgow. Pauson and Khand's initial findings were intermolecular in nature, but starting a decade after the reaction's discovery, many intramolecular examples have been highlighted in both synthesis and methodology reports. This reaction was originally mediated by stoichiometric amounts of dicobalt octacarbonyl, but newer versions are both more efficient, enhancing reactivity and yield via utilizing different chiral auxiliaries for stereo induction, main group transition-metals (Ti, Mo, W, Fe, Co, Ni, Ru, Rh, Ir and Pd), and additives. Mechanism W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cycloaddition, the 1,3-dipolar cycloaddition is a (3 + 2)-cycloaddit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enyne Metathesis
An enyne metathesis is an organic reaction taking place between an alkyne and an alkene with a metal carbene catalyst forming a butadiene. This reaction is a variation of olefin metathesis. The general scheme is given by ''scheme 1'': : When the reaction is intramolecular (in an enyne) it is called ring-closing enyne metathesis or RCEYM (''scheme 2''): : with Y representing oxygen or nitrogen and n an integer. The reaction was first described in 1985 with the conversion of biphenyl 3.1 to a phenanthrene in ''scheme 3'': : The carbene is a tungsten carbonyl when used in stoichiometric amounts (1 equivalent) yields 41% of the phenanthrene 3.2 and when used in catalytic amounts phenanthrene 3.3. The stereoselectivity of this reaction is large with the metal atom exclusively adding to one of the alkyne carbon atoms in the initial reaction step. Reaction mechanism The reaction mechanism for this reaction is outlined in scheme 4: : In the first catalytic cycle the alkyne group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as '' reagents'' or ''reactants'') that will experience a transformation when subjected to certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing (" work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the ''reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |