|
Detyrosination
Detyrosination is a form of posttranslational modification that occurs on alpha-tubulin. It consists of the removal of the C-terminus, C-terminal tyrosine to expose a glutamate at the newly formed C-terminus. Tubulin polymers, called microtubules, that contain detyrosinated alpha-tubulin are usually referred to as ''Glu-microtubules'' while unmodified polymers are called ''Tyr-microtubules''. The detyrosynating activity was first identified in the late 1970s. It is a slow acting enzyme that uses polymeric tubulin as a substrate. As a result, only stabilized microtubules accumulate this particular modification. Tubulin detyrosination is reversed by the tubulin-tyrosine ligase, which acts only on alpha-tubulin monomer. Since the majority of microtubules are very dynamic, they do not contain much detyrosinated tubulin. See also *Polyglutamylation *Polyglycylation *Acetylation References Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Aillaud ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
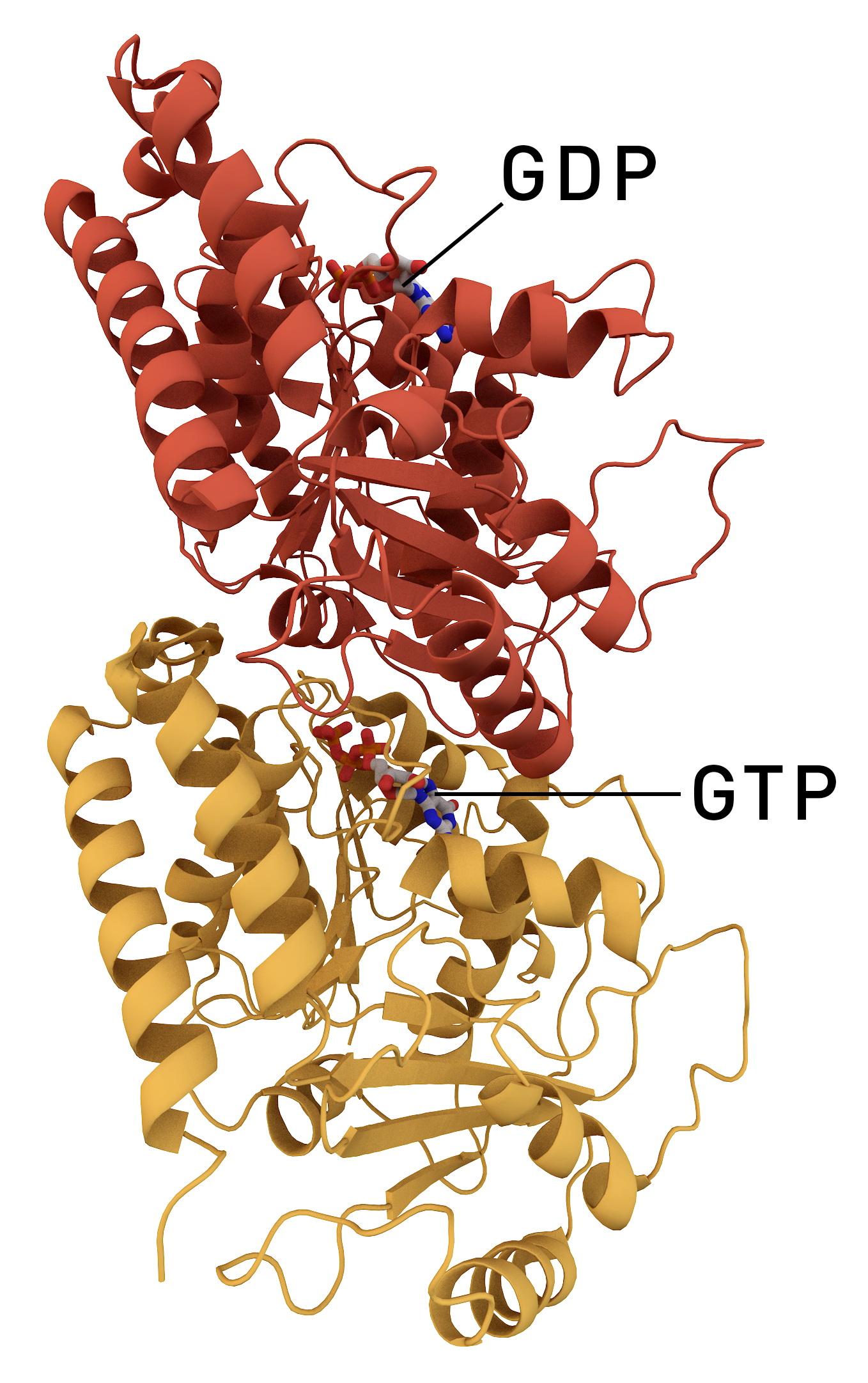
Tubulin
Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. It was discovered and named by Hideo Mōri in 1968. Microtubules function in many essential cellular processes, including mitosis. Discovery and development of tubulin inhibitors, Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division. #Eukaryotic, In eukaryotes, there are six members of the tubulin superfamily, although not all are present in all species.Turk E, Wills AA, Kwon T, Sedzinski J, Wallingford JB, Stearns "Zeta-Tubulin Is a Member of a Conserved Tubulin Module and Is a Component of the Centriolar Basal Foot in Multiciliated Cells"Current Biology (2015) 25:2177-2183. Both α and β tubulins have a mass of around 50 kDa and are thus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Microtubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nanometer, nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a Protein dimer, dimer of two globular proteins, Tubulin#Eukaryotic, alpha and beta tubulin into #Structure, protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement. Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
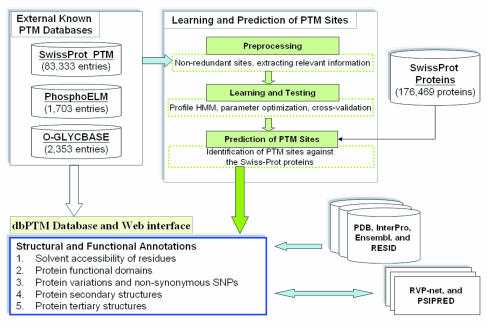
Posttranslational Modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translate mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can expand the chemical set of the 22 amino acids by changing an existing functional group or adding a new one such as phosphate. Phosphorylation is highly effective for controlling the enzyme activity and is the most common change after translation. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosylation, which can promote protein folding and improve stability a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ... or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA. The one-letter symbol Y was assigned to tyrosine for being alphabetically nearest of those letters available. Note that T was assigned to the structurally simpler threonine, U was avoided for its similarity with V for valine, W was assigned to tryptophan, while X was reserved for undetermined or atypical amino acids. The mnemonic t''Y''rosine was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Glutamate
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a Essential amino acid, non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons. Its molecular formula is . Glutamic acid exists in two optically isomeric forms; the optical rotation, dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxylic acid, carboxyl groups −COOH and one amine, amino group � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Polyglutamylation
Polyglutamylation is a form of reversible posttranslational modification of glutamate residues seen for example in alpha and beta tubulins, nucleosome assembly proteins NAP1 and NAP2. The γ-carboxy group of glutamate may form peptide-like bond with the amino group of a free glutamate whose α-carboxy group In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g. ... can now be extended into a polyglutamate chain. The glutamylation is done by the enzyme glutamylase and removed by deglutamylase. Polyglutamylation of chain length of up to six occurs in certain glutamate residues near the C terminus of most major forms of tubulins. These residues, though themselves not involved in direct binding, cause conformational shifts that regulate binding of microtubule associated proteins (MAP and Tau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Polyglycylation
Polyglycylation is a form of posttranslational modification of glutamate residues of the carboxyl-terminal region tubulin in certain microtubules (e.g., axonemal) originally discovered in ''Paramecium ''Paramecium'' ( , , plural "paramecia" only when used as a Common name, vernacular name) is a genus of eukaryotic, unicellular ciliates, widespread in freshwater, brackish, and Ocean, marine environments. Paramecia are often abundant in stagna ...'', and later shown in mammalian neurons as well. See also * Polyglutamylation References {{Protein primary structure Post-translational modification Protein structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acetylation
: In chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply ''acetates''. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound. Acetylation/deacetylation in biology Histone deacetylases "play crucial roles in gene transcription and most likely in all eukaryotic biological processes that involve chromatin". Acetylation is one type of post-translational modification of proteins. The acetylation of the ε-amino group of lysine, which is common, converts a charged side chain to a neutral one. Acetylation/deacetylation of histones also plays a role in gene expression and cancer. These modifications are effected by enzymes called histone acetyltransferases (HATs) and histone deacetylases (HDACs). Two general mechanisms are known for deacetylation. One mechanism involves zinc binding to the acetyl o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Post-translational Modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translation (biology), translate mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signal transduction, signalling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C-terminus, C- or N-terminus, N- termini. They can expand the chemical set of the 22 proteinogenic amino acid, amino acids by changing an existing functional group or adding a new one such as phosphate. Phosphorylation is highly effective for controlling the enzyme activity and is the most common change after translation. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |