|
Dehydrogenative Coupling Of Silanes
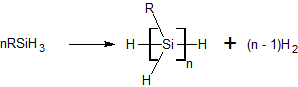
The dehydrogenative coupling of silanes is a reaction type for the formation of Si-Si bonds. Although never commercialized, the reaction has been demonstrated for the synthesis of certain disilanes as well as polysilanes. These reactions generally require catalysts. Metallocene-based catalysts Titanocene and related their complexes are typical catalysts. A typical reaction involves phenylsilane: :n PhSiH3 → hSiHsub>n + n H2 Para- and meta-substituted phenylsilanes polymerize readily but ortho-substituted polymers were failed to form. Polymers white/colorless, tacky and soluble in organic solvents. Crosslinking was not observed. Using Cp2Ti(OPh)2 as a catalyst, the dehydrogenative coupling of phenylsilane in the presence of vinyltriethoxysilane produces a polysilane terminated with a triethoxysilylvinyl group. Other catalysts The nickel(I) complex dippe)Ni(µ-H)sub>2 promotes the dehydrogenative coupling of some silanes. While catalysts for dehydrogenative couplin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
General Primary Dehydrogenative Coupling Of Primary Polysilanes
A general officer is an officer of high rank in the armies, and in some nations' air forces, space forces, and marines or naval infantry. In some usages the term "general officer" refers to a rank above colonel."general, adj. and n.". OED Online. March 2021. Oxford University Press. https://www.oed.com/view/Entry/77489?rskey=dCKrg4&result=1 (accessed May 11, 2021) The term ''general'' is used in two ways: as the generic title for all grades of general officer and as a specific rank. It originates in the 16th century, as a shortening of ''captain general'', which rank was taken from Middle French ''capitaine général''. The adjective ''general'' had been affixed to officer designations since the late medieval period to indicate relative superiority or an extended jurisdiction. Today, the title of ''general'' is known in some countries as a four-star rank. However, different countries use different systems of stars or other insignia for senior ranks. It has a NATO rank scal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disilane
Disilane is a chemical compound with chemical formula Si2H6 that was identified in 1902 by Henri Moissan and Samuel Smiles (1877–1953). Moissan and Smiles reported disilane as being among the products formed by the action of dilute acids on metal silicides. Although these reactions had been previously investigated by Friedrich Woehler and Heinrich Buff between 1857 and 1858, Moissan and Smiles were the first to explicitly identify disilane. They referred to disilane as ''silicoethane''. Higher members of the homologous series SinH2n+2 formed in these reactions were subsequently identified by Carl Somiesky (sometimes spelled "Karl Somieski") and Alfred Stock. At standard temperature and pressure, disilane is a colourless, acrid gas. Disilane and ethane have similar structures, although disilane is much more reactive. Other compounds of the general formula Si2X6 (X = hydride, halide, alkyl, aryl, and mixtures of these groups) are called disilanes. Disilane is a group 14 hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanocene
Titanocene dichloride is the organotitanium compound with the formula ( ''η''5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. It shows antitumour activity and was the first non-platinum complex to undergo clinical trials as a chemotherapy drug. Preparation and structure The standard preparations of Cp2TiCl2 start with titanium tetrachloride. The original synthesis by Wilkinson and Birmingham, using sodium cyclopentadienide, is still commonly used: :2 NaC5H5 + TiCl4 → (C5H5)2TiCl2 + 2 NaCl It can also be prepared by using freshly distilled cyclopentadiene rather than its sodium derivative: :2 C5H6 + TiCl4 → (C5H5)2TiCl2 + 2 HCl Focusing on the geometry of the Ti center, Cp2TiCl2 adopts a distorted tetrahedral geometry (counting Cp as a monodentate ligand). The Ti-Cl distance is 2.37 Å and the Cl-Ti-Cl angle is 95 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers (such as proteins). In polymer chemistry "cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties. When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies. Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. As with all science, there are overlaps, and the following delineations are a starting point to understanding the subtleties. Polymer chemistry Crosslinking is the general term for the process of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienyl
Cyclopentadienyl can refer to * Cyclopentadienyl anion, or cyclopentadienide, **Cyclopentadienyl ligand A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl anion, cyclopentadienyl groups (, abbreviated as Cp−). Cyclopentadienyl ligands almost invariably bind to metals as a hapticity, pentahapto (''η''5-) bonding ... * Cyclopentadienyl radical, • * Cyclopentadienyl cation, See also * Pentadienyl {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylsilane
Phenylsilane, also known as silylbenzene, a colorless liquid, is one of the simplest organosilanes with the formula C6 H5 SiH3. It is structurally related to toluene, with a silyl group replacing the methyl group. Both of these compounds have similar densities and boiling points due to these similarities. Phenylsilane is soluble in organic solvents. Synthesis and reactions Phenylsilane is produced in two steps from Si(OEt)4. In the first step, phenylmagnesium bromide is added to form Ph−Si(OEt)3 via a Grignard reaction. Reduction of the resulting Ph−Si(OEt)3 product with LiAlH4 affords phenylsilane. :Ph−MgBr + Si(OEt)4 → Ph−Si(OEt)3 + MgBr(OEt) :4 Ph−Si(OEt)3 + 3 LiAlH4 → 4 Ph−SiH3 + 3 LiAl(OEt)4 Uses Phenylsilane can be used to reduce tertiary phosphine oxides to the corresponding tertiary phosphine. :P(CH3)3O + PhSiH3 → P(CH3)3 + PhSiH2OH The use of phenylsilane proceeds with retention of configuration Walden inversion is the inversion of a ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyltriethoxysilane
Vinyltriethoxysilane is an organosilicon compound with the formula (C2H5O)3SiCH=CH2. It is a colorless liquid. The compound is bifunctional, featuring both a vinyl group and hydrolytically sensitive ethoxysilyl groups. As such it is a crosslinking agent.{{cite journal , doi = 10.5254/1.3538376, title = Chemical Aspects of Rubber Reinforcement by Fillers, journal = Rubber Chemistry and Technology, volume = 69, issue = 3, pages = 325–346, year = 1996, last1 = Wolff, first1 = Siegfried Applications Vinyltriethoxysilane and the related vinyltrimethoxysilane are using as monomers and comonomer for polymers such as ethylene-vinyltrimethoxysilane and ethylene-vinyl acetate-vinyltrimethoxysilane. Vinyltrialkoxysilanes are also used as cross-linking agents during the manufacture of cross-linked polyethylene (PEX). The alkoxysilane moiety is reactive toward water, and in the presence of moisture, it forms silicon-oxygen-silicon bonds that cross-link the material to cure it. Moi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from '' magnesia negra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "'' lime''" connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single chemical compound calcium oxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime. Quicklime is relatively inexpensive. Both it and a chemical derivative (calcium hydroxide, of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above ,Merck ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilkinson's Catalyst
Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula hCl(PPh3)3(Ph = phenyl). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use. Historically, Wilkinson's catalyst has been a paradigm in catalytic studies leading to several advances in the field such as the implementation of some of the first heteronuclear magnetic resonance studies for its structural elucidation in solution (31P), parahydrogen-induced polarization spectroscopy to determine the nature of transient reactive species, or one of the first detailed kinetic investigation by Halpern to elucidate the mechanism. Furthermore, the catalytic and organome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(pentafluorophenyl)borane
Tris(pentafluorophenyl)borane, sometimes referred to as "BCF", is the chemical compound . It is a white, volatile solid. The molecule consists of three pentafluorophenyl groups attached in a "paddle-wheel" manner to a central boron atom; the core is planar. It has been described as the “ideal Lewis acid” because of its high thermal stability and the relative inertness of the B-C bonds. Related fluoro-substituted boron compounds, such as those containing groups, decompose with formation of B-F bonds. Tris(pentafluorophenyl)borane is thermally stable at temperatures well over 200 °C, resistant to oxygen, and water-tolerant. Preparation Tris(pentafluorophenyl)borane is prepared using a Grignard reagent derived from bromopentafluorobenzene: : The synthesis originally employed , but this reagent can detonate with elimination of . Structure The structure of tris(pentafluorophenyl)borane (BCF) was determined by gas electron diffraction. It has a propeller-like arrangement of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |