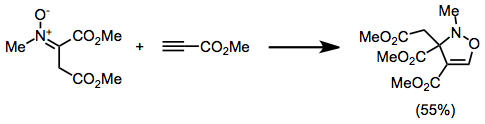|
Cyclooctyne
Cyclooctyne is the cycloalkyne with a formula . Its molecule has a ring of 8 carbon atoms, connected by seven single bonds and one triple bond. Cyclooctene is the smallest cycloalkyne that is stable enough to be isolated, although the chemical is still highly reactive. The alkyne region of the structure attempts to adopt a linear molecular geometry, but the nature of the ring creates substantial ring strain. As a result, cyclooctyne and other compounds containing this ring structure readily react in ways that reduce the ring strain by converting the alkyne to a functional group that does not require linear geometry. An important application of this reactivity is in click chemistry, where cyclooctynes undergo cycloaddition reactions with azide In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Click Chemistry
In chemical synthesis, click chemistry is a class of biocompatible small molecule reactions commonly used in bioconjugation, allowing the joining of substrates of choice with specific biomolecules. Click chemistry is not a single specific reaction, but describes a way of generating products that follow examples in nature, which also generates substances by joining small modular units. In many applications, click reactions join a biomolecule and a reporter molecule. Click chemistry is not limited to biological conditions: the concept of a "click" reaction has been used in chemoproteomic, pharmacological, and various biomimetic applications. However, they have been made notably useful in the detection, localization and qualification of biomolecules. Click reactions occur in one pot, are not disturbed by water, generate minimal and inoffensive byproducts, and are "spring-loaded"—characterized by a high thermodynamic driving force that drives it quickly and irreversibly to high y ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkyne
In organic chemistry, a cycloalkyne is the cyclic analog of an alkyne (). A cycloalkyne consists of a closed ring of carbon atoms containing one or more triple bonds. Cycloalkynes have a general formula Because of the linear nature of the alkyne unit, cycloalkynes can be highly strained and can only exist when the number of carbon atoms in the ring is great enough to provide the flexibility necessary to accommodate this geometry. Large alkyne-containing carbocycles may be virtually unstrained, while the smallest constituents of this class of molecules may experience so much strain that they have yet to be observed experimentally. Cyclooctyne () is the smallest cycloalkyne capable of being isolated and stored as a stable compound. Despite this, smaller cycloalkynes can be produced and trapped through reactions with other organic molecules or through complexation to transition metals. Background Due to the significant geometric constraints imposed by the functionality, cycloal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three isotopes occur naturally, C and C being stable, while C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity. Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen. The atoms of carbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Single Bond
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond. When shared, each of the two electrons involved is no longer in the sole possession of the orbital in which it originated. Rather, both of the two electrons spend time in either of the orbitals which overlap in the bonding process. As a Lewis structure, a single bond is denoted as AːA or A-A, for which A represents an element. In the first rendition, each dot represents a shared electron, and in the second rendition, the bar represents both of the electrons shared in the single bond. A covalent bond can also be a double bond or a triple bond. A single bond is weaker than either a double bond or a triple bond. This difference in strength can be explained by examining the component bonds of which each of these types of covalent bonds consists (Moore, Stanitsk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order of three. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are cyanides and isocyanides. Some diatomic molecules, such as dinitrogen and carbon monoxide, are also triple bonded. In skeletal formulae the triple bond is drawn as three parallel lines (≡) between the two connected atoms. Bonding The types of bonding can be explained in terms of orbital hybridization. In the case of acetylene each carbon atom has two sp-orbitals and two p-orbitals. The two sp-orbitals are linear with 180° angles and occupy the x-axis (cartesian coordinate system). The p-orbitals are perpendicular on the y-axis and the z-axis. When the carbon atoms approach each other, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Molecular Geometry
In chemistry, the linear molecular geometry describes the geometry around a central atom bonded to two other atoms (or ''ligands'') placed at a bond angle of 180°. Linear organic molecules, such as acetylene (), are often described by invoking sp orbital hybridization for their carbon centers. According to the VSEPR model (Valence Shell Electron Pair Repulsion model), linear geometry occurs at central atoms with two bonded atoms and zero or three lone pairs ( or ) in the AXE notation. Neutral molecules with linear geometry include beryllium fluoride () with two single bonds, carbon dioxide () with two double bonds, hydrogen cyanide () with one single and one triple bond. The most important linear molecule with more than three atoms is acetylene (), in which each of its carbon atoms is considered to be a central atom with a single bond to one hydrogen and a triple bond to the other carbon atom. Linear anions include azide () and thiocyanate (), and a linear cation is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring Strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated. Ring strain results from a combination of angle strain, conformational strain or Pitzer strain (torsional eclipsing interactions), and transannular strain, also known as van der Waals strain or Prelog strain. The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane. Ring strain energy can be attributed to the energy required for the distortion of bond and bond angles in order to close a ring. Ring strain energy is believed to be the cause of accelerated rates in altering ring reactions. Its interactions with traditional bond energies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cycloaddition, the 1,3-dipolar cycloaddition is a (3 + 2)-cycloaddit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant application of azides is as a propellant in air bags. Preparation Sodium azide is made industrially by the reaction of nitrous oxide, with sodium amide in liquid ammonia as solvent: : Many inorganic azides can be prepared directly or indirectly from sodium azide. For example, lead azide, used in detonators, may be prepared from the metathesis reaction between lead nitrate and sodium azide. An alternative route is direct reaction of the metal with silver azide dissolved in liquid ammonia. Some azides are produced by treating the carbonate salts with hydrazoic acid. Bonding Azide is isoelectronic with carbon dioxide , cyanate , nitrous oxide , nitronium ion and cyanogen fluoride NCF. Per valence bond theory, azide can be des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrone
In organic chemistry, a nitrone is a functional group consisting of an ''N''-oxide of an imine. The general structure is , where R’ is not a hydrogen. A nitrone is a 1,3-dipole, and is used in 1,3-dipolar cycloadditions. Other reactions of nitrones are known, including formal +3cycloadditions to form 6-membered rings, as well as formal +2cycloadditions to form 7-membered rings. Generation of nitrones Nitrones are generated most often either by the oxidation of hydroxylamines or condensation of monosubstituted hydroxylamines with carbonyl compounds (ketones or aldehydes). The most general reagent used for the oxidation of hydroxylamines is mercury(II) oxide. Carbonyl condensation methods avoid issues of site selectivity associated with the oxidation of hydroxylamines with two sets of (alpha) hydrogens. A significant problem associated with many reactive nitrones is dimerization. This issue is alleviated experimentally by employing an excess of the nitrone or increasin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
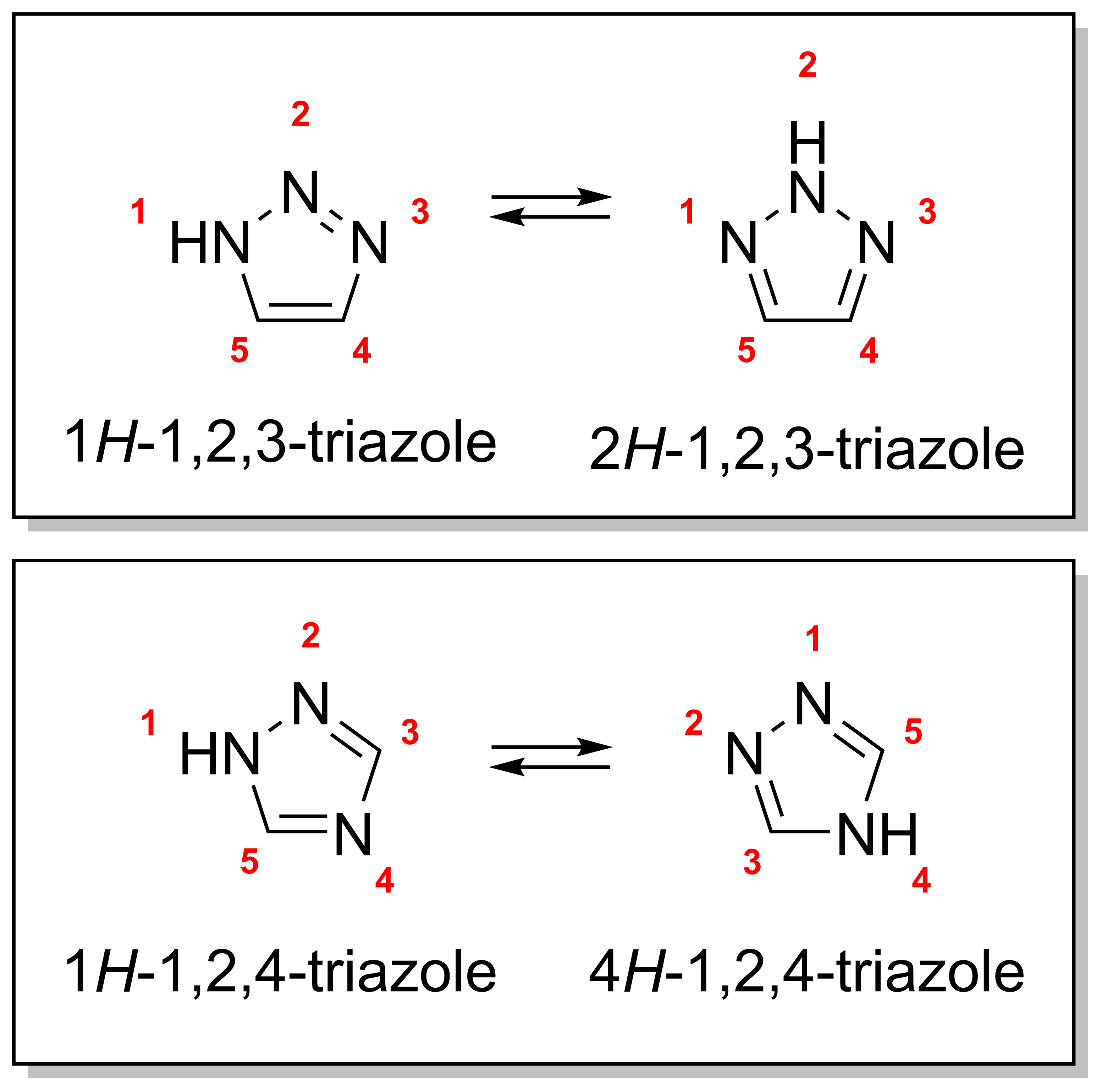
Triazole
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring. Many triazoles are versatile, biologically active compounds commonly used as fungicides and plant retardants. However, triazoles are also useful in bioorthogonal chemistry, because the large number of nitrogen atoms causes triazoles to react similar to azides. Lastly, the many free lone pairs in triazoles make them useful as coordination compounds, although not typically as haptic ligands. Isomerism There are four triazole isomers, which are conventionally divided into two pairs of tautomers. In the 1,2,3-triazoles, the three nitrogen atoms are adjacent; in the 1,2,4-triazoles, an interstitial carbon separates out one nitrogen atom. Each category has two tautomers that differ by which nitrogen has a hydrogen bonded ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoxazoline
Isoxazolines are a class of five-membered heterocyclic chemical compounds, containing one atom each of oxygen and nitrogen which are located adjacent to one another. The ring was named in-line with the Hantzsch–Widman nomenclature. They are structural isomers of the more common oxazolines and exist in three different isomers depending on the location of the double bond. The relatively weak N-O bond makes isoxazolines prone to ring-opening and rearrangement reactions. Compounds containing an isoxazoline ring, sometimes referred to isoxazolyls, have a variety of uses with many being biologically active. A number of naturally occurring isoxazolines with possible anti-cancer activity are produced by marine sponges. Perhaps the most commonly encountered products containing isoxazolines are some veterinary medicines used to prevent flea infestations in dogs e.g. Fluralaner, Afoxolaner and Sarolaner. Synthesis 2-Isoxazolines are generally produced by the 1,3-dipolar cycloaddition o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |