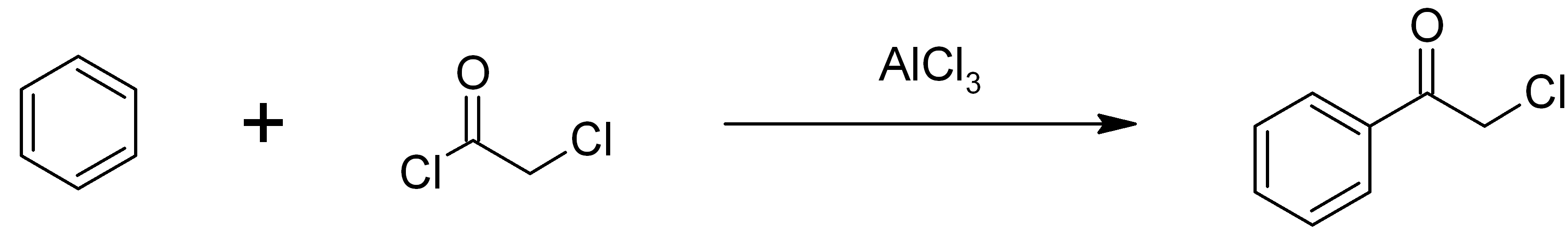|
Chloroacetyl Chloride
Chloroacetyl chloride is a chlorinated acyl chloride. It is a bifunctional compound, making it a useful building block chemical. Production Industrially, it is produced by the carbonylation of methylene chloride, oxidation of vinylidene chloride, or the addition of chlorine to ketene. It may be prepared from chloroacetic acid and thionyl chloride, phosphorus pentachloride, or phosgene. Reactions Chloroacetyl chloride is bifunctional—the acyl chloride easily forms esters and amides, while the other end of the molecule is able to form other linkages, e.g. with amines. The use of chloroacetyl chloride in the synthesis of lidocaine is illustrative: : Applications The major use of chloroacetyl chloride is as an intermediate in the production of herbicides in the chloroacetanilide family including metolachlor, acetochlor, alachlor and butachlor; an estimated 100 million pounds are used annually. Some chloroacetyl chloride is also used to produce phenacyl chloride, another chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl Chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example of an acyl chloride is acetyl chloride, . Acyl chlorides are the most important subset of acyl halides. Nomenclature Where the acyl chloride Moiety (chemistry), moiety takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid, and substituting ''-yl chloride'' for ''-ic acid''. Thus: : : When other functional groups take priority, acyl chlorides are considered prefixes — ''chlorocarbonyl-'': : Properties Lacking the ability to form hydrogen bonds, acyl chlorides have lower boiling and melting points than similar carboxylic acids. For example, acetic acid boils at 118 °C, whereas acetyl chloride boils at 51 °C. Like most carbonyl compounds, infrared spectroscopy reveals a band near 1750 cm� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metolachlor
Metolachlor is an organic compound that is widely used as an herbicide. It is a derivative of aniline and is a member of the chloroacetanilide family of herbicides. It is highly effective toward grasses. Agricultural use Metolachlor was developed by Ciba-Geigy. Its acts by inhibition of elongases and of the geranylgeranyl pyrophosphate (GGPP) cyclases, which are part of the gibberellin pathway. It is used for grass and broadleaf weed control in corn, soybean, peanuts, sorghum, and cotton. It is also used in combination with other herbicides. Metolachlor is a popular herbicide in the United States. As originally formulated metolachlor was applied as a racemate, a 1:1 mixture of the (''S'')- and (''R'')-stereoisomers. The (''R'')-enantiomer is less active, and modern production methods afford a higher concentration of S-metolachlor, thus current application rates are far lower than original formulations. Production and basic structure Metolachlor is produced from 2-ethyl-6- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lachrymator
Tear gas, also known as a lachrymator agent or lachrymator (), sometimes colloquially known as "mace" after the early commercial aerosol, is a chemical weapon that stimulates the nerves of the lacrimal gland in the eye to produce tears. In addition, it can cause severe eye and respiratory pain, skin irritation, bleeding, and blindness. Common lachrymators both currently and formerly used as tear gas include pepper spray (OC gas), PAVA spray ( nonivamide), CS gas, CR gas, CN gas (phenacyl chloride), bromoacetone, xylyl bromide and Mace (a branded mixture). While lachrymatory agents are commonly deployed for riot control by law enforcement and military personnel, its use in warfare is prohibited by various international treaties.E.g. the Geneva Protocol of 1925 prohibited the use of "asphyxiating gas, or any other kind of gas, liquids, substances or similar materials". During World War I, increasingly toxic and deadly lachrymatory agents were used. The short and long-te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Venlafaxine
Venlafaxine, sold under the brand name Effexor among others, is an antidepressant medication of the serotonin-norepinephrine reuptake inhibitor (SNRI) class. It is used to treat major depressive disorder, generalized anxiety disorder, panic disorder, and social anxiety disorder. It may also be used for chronic pain. It is taken by mouth. It is also available as the salt venlafaxine besylate in an extended-release formulation (Venbysi XR). Common side effects include loss of appetite, constipation, dry mouth, dizziness, sweating, insomnia, drowsiness and sexual problems. Severe side effects include an increased risk of suicide, mania, and serotonin syndrome. Antidepressant withdrawal syndrome may occur if stopped. There are concerns that use during the later part of pregnancy can harm the baby. How it works is not entirely clear, but it seems to be related to the potentiation of the activity of some neurotransmitters in the brain. Venlafaxine was approved for medical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anisole
Anisole, or methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound is mainly made synthetically and is a precursor to other synthetic compounds. It is an ether. Anisole is a standard reagent of both practical and pedagogical value. It can be prepared by the Williamson ether synthesis; sodium phenoxide is reacted with a methyl halide to yield anisole. Reactivity Anisole undergoes electrophilic aromatic substitution reaction at a faster speed than benzene, which in turn reacts more quickly than nitrobenzene. The methoxy group is an ortho/para directing group, which means that electrophilic substitution preferentially occurs at these three sites. The enhanced nucleophilicity of anisole vs. benzene reflects the influence of the methoxy group, which renders the ring more electron-rich. The methoxy group s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Preparation Of Phenacyl Chloride
Preparation may refer to: * Preparation (dental), the method by which a tooth is prepared when removing decay and designing a form that will provide adequate retention for a dental restoration * Preparation (music) In tonal music, a preparation is the consonant pitch or chord which precedes a dissonant nonharmonic tone. The move from a dissonance to a consonance constitutes a resolution. In the following example, the C major chord on the left is a preparat ..., treatment of dissonance in tonal music * Preparation, Iowa, a ghost town * Preparation time, time to prepare speeches in policy debate * ''The Preparation'', a 2017 South Korean film * Preparations (album), ''Preparations'' (album), a 2007 album by Prefuse 73 * Prepared dosage form * Prepared drug * Prepared food * Prepared Dietary supplement, supplement * Special modifications to instruments, see **Prepared piano **Prepared guitar *Fossil preparation See also * Preparation H, popular hemorrhoids medicine * Preparat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms hexahydrate with the formula , containing six water molecules of hydration. Both are colourless crystals, but samples are often contaminated with iron(III) chloride, giving a yellow color. The anhydrous material is important commercially. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry. The compound is often cited as a Lewis acid. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. Structure Anhydrous adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geometry. In contrast, has a more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" ( ben ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenacyl Chloride
Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN. It should not be confused with cyanide, another agent used in chemical warfare, which has the chemical structure CN−. Preparation Phenacyl chloride is readily available and was first prepared by chlorination of acetophenone vapour. It may also be synthesized by the Friedel-Crafts acylation of benzene using chloroacetyl chloride, with an aluminium chloride catalyst: Riot control agent It was investigated, but not used, during the First and Second World Wars. Because of its significantly greater toxicity, it has largely been supplanted by CS gas. Even though CN is still supplied to paramilitary and police forces in a small pressurized aerosol known as “ Mace” or tear gas, its use is falling as pepper spray both works and dispe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenacyl Chloride
Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN. It should not be confused with cyanide, another agent used in chemical warfare, which has the chemical structure CN−. Preparation Phenacyl chloride is readily available and was first prepared by chlorination of acetophenone vapour. It may also be synthesized by the Friedel-Crafts acylation of benzene using chloroacetyl chloride, with an aluminium chloride catalyst: Riot control agent It was investigated, but not used, during the First and Second World Wars. Because of its significantly greater toxicity, it has largely been supplanted by CS gas. Even though CN is still supplied to paramilitary and police forces in a small pressurized aerosol known as “ Mace” or tear gas, its use is falling as pepper spray both works and dispe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)

2.png)