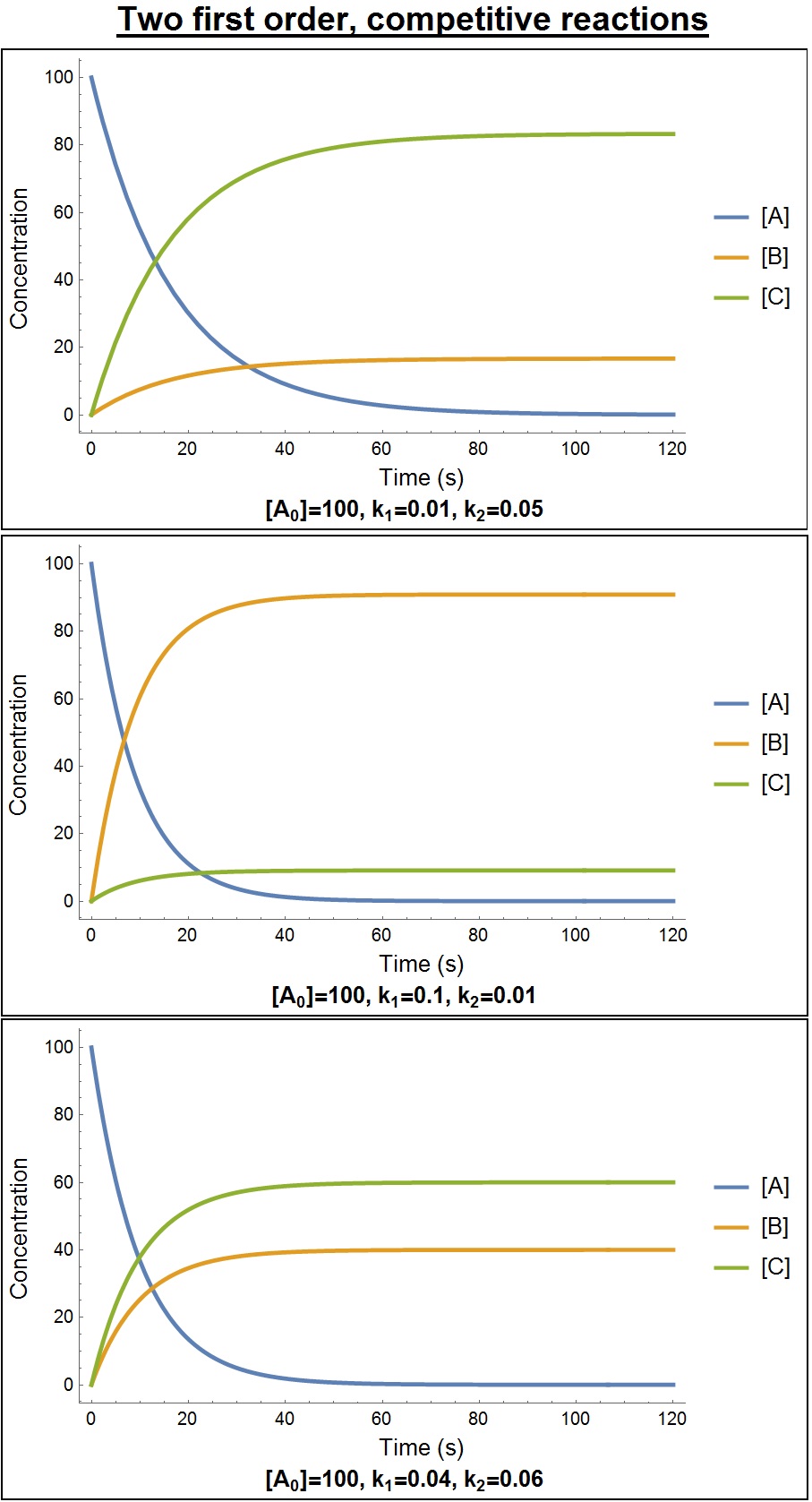
|
Chemical Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible, and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, and which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work ( pharmacology), and how to collec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. In molecular dynamics simulations, a reaction coordinate is called collective variable. These coordinates can sometimes represent a real coordinate system (such as bond length, bond angle...), although, for more complex reactions especially, this can be difficult (and non geometric parameters are used, e.g., bond order). Reaction coordinate is distinct from extent of reaction, a different parameter of reaction progress, which is a measure of the ''composition'' of the reaction system. (Free) energy is often plotted against reaction coordinate(s) to demonstrate in some schematic form the potential energy profile (an intersection of a potential energy surface) associated with the reaction. In the formalism of transition-state theory the reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. Photodissociation is not limited to visible light. Any photon with sufficient energy can affect the chemical bonds of a chemical compound. Since a photon's energy is inversely proportional to its wavelength, electromagnetic radiations with the energy of visible light or higher, such as ultraviolet light, x-rays, and gamma rays can induce such reactions. Photolysis in photosynthesis Photolysis is part of the light-dependent reaction or light phase or photochemical phase or Hill reaction of photosynthesis. The general reaction of photosynthetic photolysis can be given in terms of photons as: :\ce + 2 \text \longrightarrow \ce The chemical nature of "A" depends on the type of organism. Purple sulfur bacteria oxidize hydrogen sulfide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermolysis
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction. Decomposition temperature definition A simple substance (like water) may exist in equilibrium with its thermal decomposition products, effectively halting the decomposition. The equilibrium fraction of decomposed molecules increases with the temperature. Examples * Calcium carbonate (limestone or chalk) decomposes into calcium oxide and carbon dioxide when heated. The chemical reaction is as follows: ::CaCO3 → CaO + CO2 :The reaction is used to make quick lime, which is an industrially important prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chain Propagation
Chain propagation (sometimes referred to as propagation) is a process in which a reactive intermediate is continuously regenerated during the course of a chemical chain reaction. For example, in the chlorination of methane, there is a two-step propagation cycle involving as chain carriers a chlorine atom and a methyl radical IUPAC Gold Book which are regenerated alternately: :•Cl + CH4 → HCl + •CH3 :•CH3 + Cl2 → CH3Cl + •Cl The two steps add to give the equation for the overall chain reaction: :CH4 + Cl2 → CH3Cl + HCl. Polymerization In a |
Chain Reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events. Chain reactions are one way that systems which are not in thermodynamic equilibrium can release energy or increase entropy in order to reach a state of higher entropy. For example, a system may not be able to reach a lower energy state by releasing energy into the environment, because it is hindered or prevented in some way from taking the path that will result in the energy release. If a reaction results in a small energy release making way for more energy releases in an expanding chain, then the system will typically collapse explosively until much or all of the stored energy has been released. A macroscopic metaphor for chain reactions is thus a snowball causing a larger snowball until finally an avalanche results (" snowball effect"). This is a result of stored gravitat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoin Condensation2
Benzoin may refer to: *Benzoin (organic compound), an organic compound with the formula PhCH(OH)C(O)Ph *Benzoin (resin), a balsamic resin obtained from the bark of several species of trees in the genus Styrax * Benzoin aldolase, an enzyme that catalyzes the chemical reaction benzoin to benzaldehyde *Benzoin condensation, a reaction between two aromatic aldehydes * Benzoin odoriferum or Lindera benzoin, a shrub in the laurel family * Benzoin tree, the common name of Styrax, a genus of shrubs or trees in the family Styracaceae *Tincture of benzoin, a pungent solution of benzoin resin in ethanol See also * C14H12O2, molecular formula of benzoin *Benzene (C6H6), organic chemical compound of hydrocarbon class *Benzoic acid Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, wh ... (or C6H5 [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (inclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecularity
In chemistry, molecularity is the number of molecules that come together to react in an elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of stoichiometric coefficients of reactants in the elementary reaction with effective collision ( sufficient energy) and correct orientation. Depending on how many molecules come together, a reaction can be unimolecular, bimolecular or even trimolecular. The kinetic order of any elementary reaction or reaction step is ''equal'' to its molecularity, and the rate equation of an elementary reaction can therefore be determined by inspection, from the molecularity. The kinetic order of a complex (multistep) reaction, however, is not necessarily equal to the number of molecules involved. The concept of molecularity is only useful to describe elementary reactions or steps. Unimolecular reactions In a unimolecular reaction, a single molecule rearranges atoms, formi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate-determining Step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the corresponding rate equation (for comparison with the experimental rate law) is often simplified by using this approximation of the rate-determining step. In principle, the time evolution of the reactant and product concentrations can be determined from the set of simultaneous rate equations for the individual steps of the mechanism, one for each step. However, the analytical solution of these differential equations is not always easy, and in some cases numerical integration may even be required. The hypothesis of a single rate-determining step can greatly simplify the mathematics. In the simplest case the initial step is the slowest, and the overall rate is just the rate of the first step. Also, the rate equations for mechanisms with a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate Law
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction orders). For many reactions, the initial rate is given by a power law such as :v_0\; =\; k mathrmx mathrmy where and express the concentration of the species and usually in moles per liter ( molarity, ). The exponents and are the partial ''orders of reaction'' for and and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The constant is the reaction rate constant or ''rate coefficient'' of the reaction. Its value may depend on conditions such as temperature, ionic strength, surface area of an adsorbent, or light irradiation. If the reaction goes to completion, the rate equation for the reaction rate v\; =\; k cex cey applies throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Order
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction orders). For many reactions, the initial rate is given by a power law such as :v_0\; =\; k mathrmx mathrmy where and express the concentration of the species and usually in moles per liter (molarity, ). The exponents and are the partial ''orders of reaction'' for and and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The constant is the reaction rate constant or ''rate coefficient'' of the reaction. Its value may depend on conditions such as temperature, ionic strength, surface area of an adsorbent, or light irradiation. If the reaction goes to completion, the rate equation for the reaction rate v\; =\; k cex cey applies thr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |