|
Carborane Superacid
Carborane acids (X, Y, Z = H, Alk, F, Cl, Br, CF3) are a class of superacids, some of which are estimated to be at least one million times stronger than 100% pure sulfuric acid in terms of their Hammett acidity function values (''H''0 ≤ –18) and possess computed p''K''a values well below –20, establishing them as some of the strongest known Brønsted acids. The best-studied example is the highly chlorinated derivative . The acidity of was found to vastly exceed that of triflic acid, , and bistriflimide, , compounds previously regarded as the strongest isolable acids. Their high acidities stem from the extensive delocalization of their conjugate bases, carboranate anions (CXB11Y5Z6−), which are usually further stabilized by electronegative groups like Cl, F, and CF3. Due to the lack of oxidizing properties and the exceptionally low nucleophilicity and high stability of their conjugate bases, they are the only superacids known to protonate C60 fullerene without decomposin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophilic Aromatic Substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, and alkylation and acylation Friedel–Crafts reaction. Illustrative reactions The most widely practised example of this reaction is the ethylation of benzene. :: Approximately 24,700,000 tons were produced in 1999. (After dehydrogenation and polymerization, the commodity plastic polystyrene is produced.) In this process, acids are used as catalyst to generate the incipient carbocation. Many other electrophilic reactions of benzene are conducted, although on a much smaller scale; they are valuable routes to key intermediates. The nitration of benzene is achieved via the action of the nitronium ion as the electrophile. The sulfonation with fuming sulfuric acid gives benzenesul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoboron Compounds
Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds. Organoboron compounds are important reagents in organic chemistry enabling many chemical transformations, the most important one called hydroboration. Reactions of organoborates and boranes involve the transfer of a nucleophilic group attached to boron to an electrophilic center either inter- or intramolecularly. α,β-Unsaturated borates, as well as borates with a leaving group at the α position, are highly susceptible to intramolecular 1,2-migration of a group from boron to the electrophilic α position. Oxidation or protonolysis of the resulting organoboranes may generate a variety of organic products, including alcohols, carbonyl compounds, alkenes, and halides. Properties of the B-C bond The C-B bond has low polarity (the difference in electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decaborane
Decaborane, also called decaborane(14), is the borane with the chemical formula B10 H14. This white crystalline compound is one of the principal boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides. It is toxic and volatile, with a foul odor. Handling, properties and structure The physical characteristics of decaborane(14) resemble those of naphthalene and anthracene, all three of which are volatile colorless solids. Sublimation is the common method of purification. Decaborane is highly flammable, but, like other boron hydrides, it burns with a bright green flame. It is not sensitive to moist air, although it hydrolyzes in boiling water, releasing hydrogen and giving a solution of boric acid. It is soluble in cold water as well as a variety of non-polar and moderately polar solvents. In decaborane, the B10 framework resembles an incomplete octadecahedron. Each boron has one "radial" hydride, and four boron atoms near the open part of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carborane Figure
Carboranes are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these clusters are polyhedra or fragments of polyhedra. Carboranes are one class of heteroboranes. In terms of scope, carboranes can have as few as 5 and as many as 14 atoms in the cage framework. The majority have two cage carbon atoms. The corresponding C-alkyl and B-alkyl analogues are also known in a few cases. Structure and bonding Carboranes and boranes adopt 3-dimensional cage (cluster) geometries in sharp contrast to typical organic compounds. Cages are compatible with sigma—delocalized bonding, whereas hydrocarbons are typically chains or rings. Like for other electron-delocalized polyhedral clusters, the electronic structure of these cluster compounds can be described by the Wade–Mingos rules. Like the related boron hydrides, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas-phase Acidity
The proton affinity (PA, ''E''pa) of an anion or of a neutral atom or molecule is the negative of the enthalpy change in the reaction between the chemical species concerned and a proton in the gas phase: ::: A- + H+ -> HA ::: B + H+ -> BH+ These reactions are always exothermic in the gas phase, i.e. energy is released (enthalpy is negative) when the reaction advances in the direction shown above, while the proton affinity is positive. This is the same sign convention used for electron affinity. The property related to the proton affinity is the gas-phase basicity, which is the negative of the Gibbs energy for above reactions, i.e. the gas-phase basicity includes entropic terms in contrast to the proton affinity. Acid/base chemistry The higher the proton affinity, the stronger the base and the weaker the conjugate acid ''in the gas phase''. The (reportedly) strongest known base is the ortho-diethynylbenzene dianion (''E''pa = 1843 kJ/mol), followed by the methanid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picric Acid
Picric acid is an organic compound with the formula (O2N)3C6H2OH. Its IUPAC name is 2,4,6-trinitrophenol (TNP). The name "picric" comes from el, πικρός (''pikros''), meaning "bitter", due to its bitter taste. It is one of the most acidic phenols. Like other strongly nitrated organic compounds, picric acid is an explosive, which is its primary use. It has also been used as medicine (antiseptic, burn treatments) and as a dye. History Picric acid was probably first mentioned in the alchemical writings of Johann Rudolf Glauber. Initially, it was made by nitrating substances such as animal horn, silk, indigo, and natural resin, the synthesis from indigo first being performed by Peter Woulfe during 1771. The German chemist Justus von Liebig had named picric acid (rendered in French as ). Picric acid was given that name by the French chemist Jean-Baptiste Dumas in 1841. Its synthesis from phenol, and the correct determination of its formula, were accomplished during 1841. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroantimonic Acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This substance is a superacid that can be over a billion times stronger than 100% pure sulfuric acid in terms of its protonating ability measured by Hammett function. It even protonates some hydrocarbons to afford pentacoordinate carbocations ( carbonium ions). Fluoroantimonic acid is corrosive. For example, it cannot be contained directly in glass carboys, as it attacks glass, but can be stored in containers lined with PTFE (Teflon). Chemical composition Fluoroantimonic acid is formed by combining hydrogen fluoride and antimony pentafluoride: :SbF5 + 2 HF + H2F+ The speciation (i.e., the inventory of components) of "fluoroantimonic acid" is complex. Spectroscopic measurements show that fluoroantimonic acid consists of a mixture of HF-solvated protons, –_(such_as_)._Thus,_the_formula_""_is_a_convenient_but_oversi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
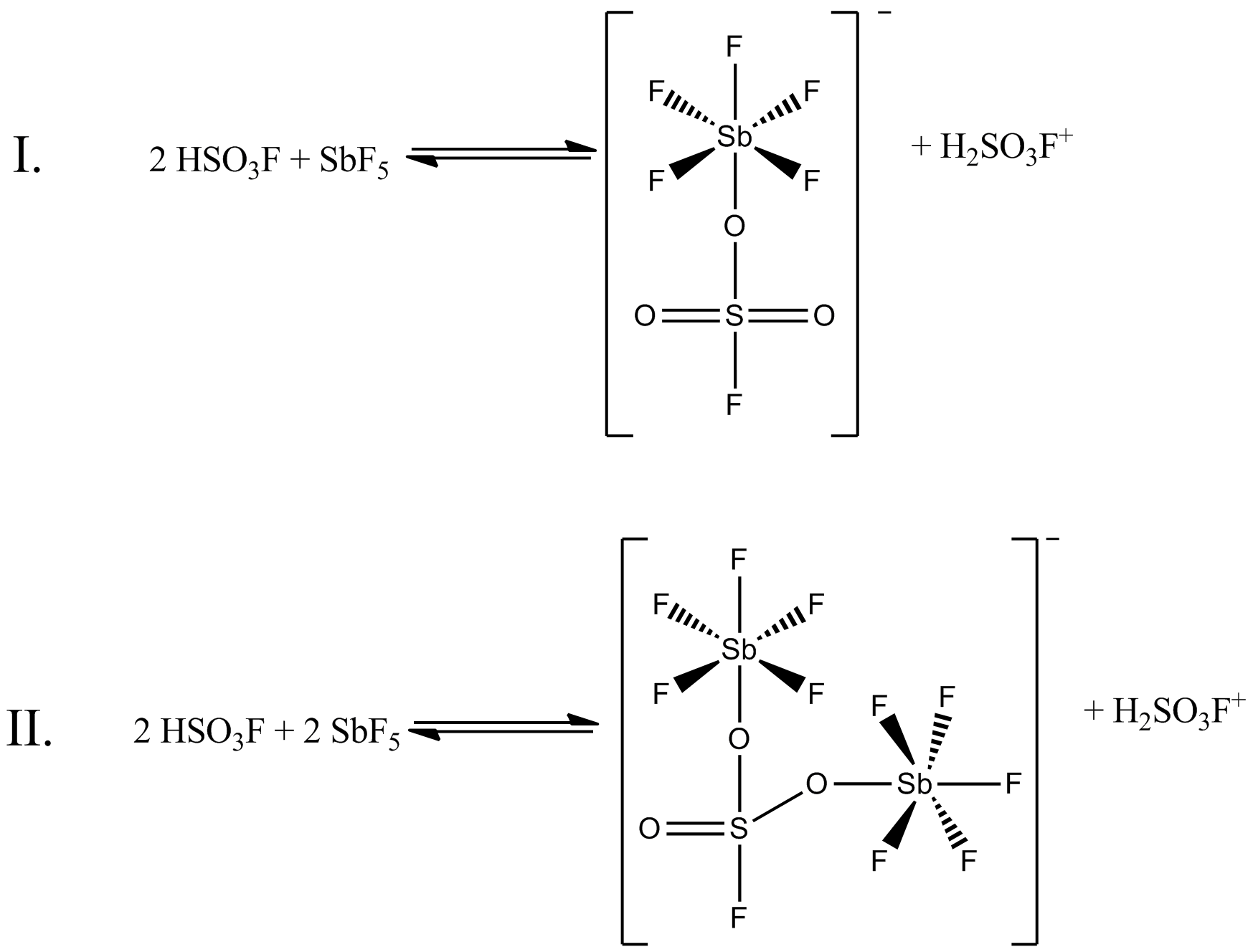
Magic Acid
Magic acid (FSO3H·SbF5) is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). This conjugate Brønsted– Lewis superacid system was developed in the 1960s by the George Olah lab at Case Western Reserve University, and has been used to stabilize carbocations and hypercoordinated carbonium ions in liquid media. Magic acid and other superacids are also used to catalyze isomerization of saturated hydrocarbons, and have been shown to protonate even weak bases, including methane, xenon, halogens, and molecular hydrogen. History The term "superacid" was first used in 1927 when James Bryant Conant found that perchloric acid could protonate ketones and aldehydes to form salts in nonaqueous solution. The term itself was coined by R. J. Gillespie later, after Conant combined sulfuric acid with fluorosulfuric acid, and found the solution to be several million times more acidic than sulfuric a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |