|
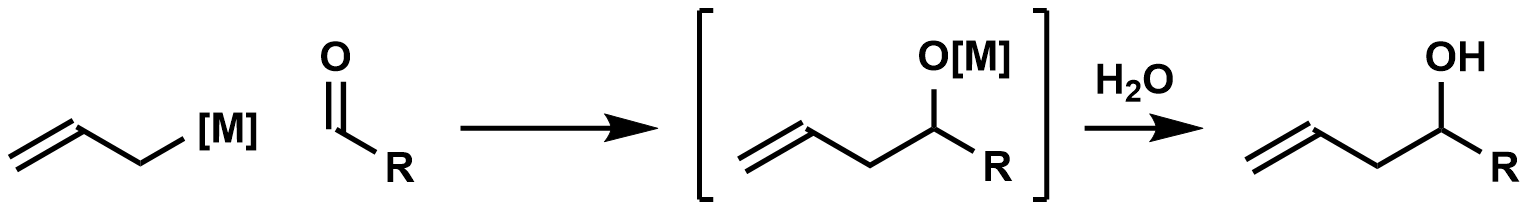
Carbonyl Allylation
In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent. Enantioselective versions In 1978, Hoffmann reported the first asymmetric carbonyl allylation using a chiral allylmetal reagent, an allylborane derived from camphor. Such methods utilize preformed allyl metal reagents. The approach is well developed using allyl boranesDenmark, S. E.; Almstead, N. G. In ''Modern Carbonyl Chemistry''; Otera, J., Ed.; Wiley-VCH: Weinheim, 2000; Chapter 10. As illustrated by the Keck allylation, catalytic enantioselective additions of achiral allylmetal reagents to carbonyl compounds also are possible by organostannane additions. Allylic boronate and -borane reagents have also been developed for enantioselective addition to carbonyls—in this class of reactions, the allylic boron reagent confers ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nozaki–Hiyama–Kishi Reaction
The Nozaki–Hiyama–Kishi reaction is a nickel/ chromium coupling reaction forming an alcohol from the reaction of an aldehyde with an allyl or vinyl halide. In their original 1977 publication, Tamejiro Hiyama and Hitoshi Nozaki reported on a chromium(II) salt solution prepared by reduction of chromic chloride by lithium aluminium hydride to which was added benzaldehyde and allyl chloride: : Compared to Grignard reactions, this reaction is very selective towards aldehydes with large tolerance towards a range of functional groups such as ketones, esters, amides and nitriles. Enals give exclusively 1,2-addition. Solvents of choice are DMF and DMSO, one solvent requirement is solubility of the chromium salts. Nozaki–Hiyama–Kishi reaction is a useful method for preparing medium-size rings. In 1983 the scope was extended by the same authors to include vinyl halides or triflates and aryl halides. It was observed that the success of the reaction depended on the source o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Allylation Scheme 3
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sirolimus
Sirolimus, also known as rapamycin and sold under the brand name Rapamune among others, is a macrolide compound that is used to coat coronary stents, prevent organ transplant rejection, treat a rare lung disease called lymphangioleiomyomatosis, and treat perivascular epithelioid cell tumor ( PEComa). It has immunosuppressant functions in humans and is especially useful in preventing the rejection of kidney transplants. It is a mechanistic target of rapamycin kinase (mTOR) inhibitor that inhibits activation of T cells and B cells by reducing their sensitivity to interleukin-2 (IL-2). It is produced by the bacterium ''Streptomyces hygroscopicus'' and was isolated for the first time in 1972, from samples of ''Streptomyces hygroscopicus'' found on Easter Island. The compound was originally named rapamycin after the native name of the island, Rapa Nui. Sirolimus was initially developed as an antifungal agent. However, this use was abandoned when it was discovered to have potent im ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrolide
The Macrolides are a class of natural products that consist of a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. The lactone rings are usually 14-, 15-, or 16-membered. Macrolides belong to the polyketide class of natural products. Some macrolides have antibiotic or antifungal activity and are used as pharmaceutical drugs. Rapamycin is also a macrolide and was originally developed as an antifungal, but is now used as an immunosuppressant drug and is being investigated as a potential longevity therapeutic. Macrolides are bacteriostatic in that they suppress or inhibit bacterial growth rather than killing bacteria completely. Definition In general, any macrocyclic lactone having greater than 8-membered rings are candidates for this class. The macrocycle may contain amino nitrogen, amide nitrogen (but should be differentiated from cyclopeptides), an oxazole ring, or a thiazole ring. Benzene rings are e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyketide
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity. History Naturally produced polyketides by various plants and organisms have been used by humans since before studies on them began in the 19th and 20th century. In 1893, J. Norman Collie synthesized detectable amounts of orcinol by heating d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Allylation Scheme 2
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Acetate
Allyl acetate is an organic compound with formula C3H5OC(O)CH3. This colourless liquid is a precursor to especially allyl alcohol, which is a useful industrial intermediate. It is the acetate ester of allyl alcohol. Preparation Allyl acetate is produced industrially by the gas phase reaction of propene in the presence of acetic acid using a palladium catalyst: :C3H6 + CH3COOH + ½ O2 → CH2=CHCH2OCOCH3 + H2O This method is advantageous because propene is inexpensive and "green." Allyl alcohol is also produced primarily from allyl chloride, but production via the hydrolysis of allyl acetate route avoids the use of chlorine, and so is increasing in use. Vinyl acetate is produced similarly, using ethylene in place of propene. These reactions are examples of acetoxylation. The palladium center is then re-oxidized by the O2 present. The mechanism for the acetoxylation follows a similar pathway, with propene forming a π-allyl bond on the palladium. : Reactions and applica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Krische Allylation
The Krische allylation involves the enantioselective iridium-catalyzed addition of an allyl group to an aldehyde or an alcohol, resulting in the formation of a secondary homoallylic alcohol. The mechanism of the Krische allylation involves primary alcohol dehydrogenation or, when using aldehyde reactants, hydrogen transfer from 2-propanol. Unlike other allylation methods, the Krische allylation avoids the use of preformed allyl metal reagents and enables the direct conversion of primary alcohols to secondary homoallylic alcohols (precluding alcohol to aldehyde oxidation). Background Enantioselective carbonyl allylations are frequently applied to the synthesis of polyketide natural products. In 1978, Hoffmann reported the first asymmetric carbonyl allylation using a chiral allylmetal reagent, an allylborane derived from camphor. Subsequently, other chiral allylmetal reagents were developed by Kumada, Roush, Brown, Leighton, and others. These methods utilize preformed allyl meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyltrimethylsilane
Allyltrimethylsilane is the organosilicon compound Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic c ... with the formula (CH3)3SiCH2CH=CH2. The molecule consists of the trimethylsilyl group attached to allyl group. This colorless liquid is used in organic synthesis.{{cite journal , doi=10.15227/orgsyn.062.0086, title=Conjugate Allylation of α,β-Unsaturated Ketones with Allylsilanes: 4-Phenyl-6-Hepten-2-one , journal=Organic Syntheses , year=1984 , volume=62 , page=86, author=Hideki Sakurai, Akira Hosomi, Josabro Hayashi References Reagents for organic chemistry Trimethylsilyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hisashi Yamamoto
(born July 16, 1943) is a prominent organic chemist and currently a member of the faculty at the University of Chicago and professor of Chubu University. Life Born in Kobe, Japan, Yamamoto earned a B.S. at Kyoto University in 1967 and a Ph.D. at Harvard University in 1971. He was a professor at Nagoya University from 1983 until 2002 and has since been a professor within the Department of Chemistry at the University of Chicago. His research work is largely in the chemistry of acid catalysts that play an important role in triggering or driving chemical reactions, specifically Lewis and Brønsted acid catalysts used in selective organic synthesis. Yamamoto has authored or co-authored several books on topics in modern synthetic organic chemistry. As of 2021, his h-index equals to 120 with more than 64,000 citations. Awards and recognitions *1988 Japan IBM Science Prize *1992 Chu-Nichi Culture Prize *2002 Medals with Purple ribbon *2003 Fellow of the American Association for t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |