|
Canonical Ensemble
In statistical mechanics, a canonical ensemble is the statistical ensemble that represents the possible states of a mechanical system in thermal equilibrium with a heat bath at a fixed temperature. The system can exchange energy with the heat bath, so that the states of the system will differ in total energy. The principal thermodynamic variable of the canonical ensemble, determining the probability distribution of states, is the absolute temperature (symbol: ). The ensemble typically also depends on mechanical variables such as the number of particles in the system (symbol: ) and the system's volume (symbol: ), each of which influence the nature of the system's internal states. An ensemble with these three parameters, which are assumed constant for the ensemble to be considered canonical, is sometimes called the ensemble. The canonical ensemble assigns a probability to each distinct microstate given by the following exponential: :P = e^, where is the total energy of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Microcanonical Ensemble
In statistical mechanics, the microcanonical ensemble is a statistical ensemble that represents the possible states of a mechanical system whose total energy is exactly specified. The system is assumed to be isolated in the sense that it cannot exchange energy or particles with its environment, so that (by conservation of energy) the energy of the system does not change with time. The primary macroscopic variables of the microcanonical ensemble are the total number of particles in the system (symbol: ), the system's volume (symbol: ), as well as the total energy in the system (symbol: ). Each of these is assumed to be constant in the ensemble. For this reason, the microcanonical ensemble is sometimes called the ensemble. In simple terms, the microcanonical ensemble is defined by assigning an equal probability to every microstate whose energy falls within a range centered at . All other microstates are given a probability of zero. Since the probabilities must add up to 1, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Statistical Mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. Sometimes called statistical physics or statistical thermodynamics, its applications include many problems in a wide variety of fields such as biology, neuroscience, computer science Computer science is the study of computation, information, and automation. Computer science spans Theoretical computer science, theoretical disciplines (such as algorithms, theory of computation, and information theory) to Applied science, ..., information theory and sociology. Its main purpose is to clarify the properties of matter in aggregate, in terms of physical laws governing atomic motion. Statistical mechanics arose out of the development of classical thermodynamics, a field for which it was successful in explaining macroscopic physical properties—such as temperature, pressure, and heat capacity—in terms of microscop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Thermodynamic Limit
In statistical mechanics, the thermodynamic limit or macroscopic limit, of a system is the Limit (mathematics), limit for a large number of particles (e.g., atoms or molecules) where the volume is taken to grow in proportion with the number of particles.S.J. Blundell and K.M. Blundell, "Concepts in Thermal Physics", Oxford University Press (2009) The thermodynamic limit is defined as the limit of a system with a large volume, with the particle density held fixed: : N \to \infty,\, V \to \infty,\, \frac N V =\text In this limit, macroscopic thermodynamics is valid. There, thermal fluctuations in global quantities are negligible, and all List of thermodynamic properties, thermodynamic quantities, such as pressure and energy, are simply functions of the thermodynamic variables, such as temperature and density. For example, for a large volume of gas, the fluctuations of the total internal energy are negligible and can be ignored, and the average internal energy can be predicted fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Maxwell Speed Distribution
Maxwell may refer to: People * Maxwell (surname), including a list of people and fictional characters with the name ** James Clerk Maxwell, mathematician and physicist * Justice Maxwell (other) * Maxwell baronets, in the Baronetage of Nova Scotia * Maxwell (footballer, born 1979), Brazilian forward * Maxwell (footballer, born 1981), Brazilian left-back * Maxwell (footballer, born 1986), Brazilian striker * Maxwell (footballer, born 1989), Brazilian left-back * Maxwell (footballer, born 1995), Brazilian forward * Maxwell (musician) (born 1973), American R&B and neo-soul singer * Maxwell (rapper) (born 1993), German rapper, member of rap band 187 Strassenbande * Maxwell Jacob Friedman (born 1996), American professional wrestler * Maxwell Silva (born 1953), Sri Lankan Sinhala Catholic cleric, Auxiliary Bishop of Colombo Places United States * Maxwell, California * Maxwell, Indiana * Maxwell, Iowa * Maxwell, Nebraska * Maxwell, New Mexico * Maxwell, Texas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
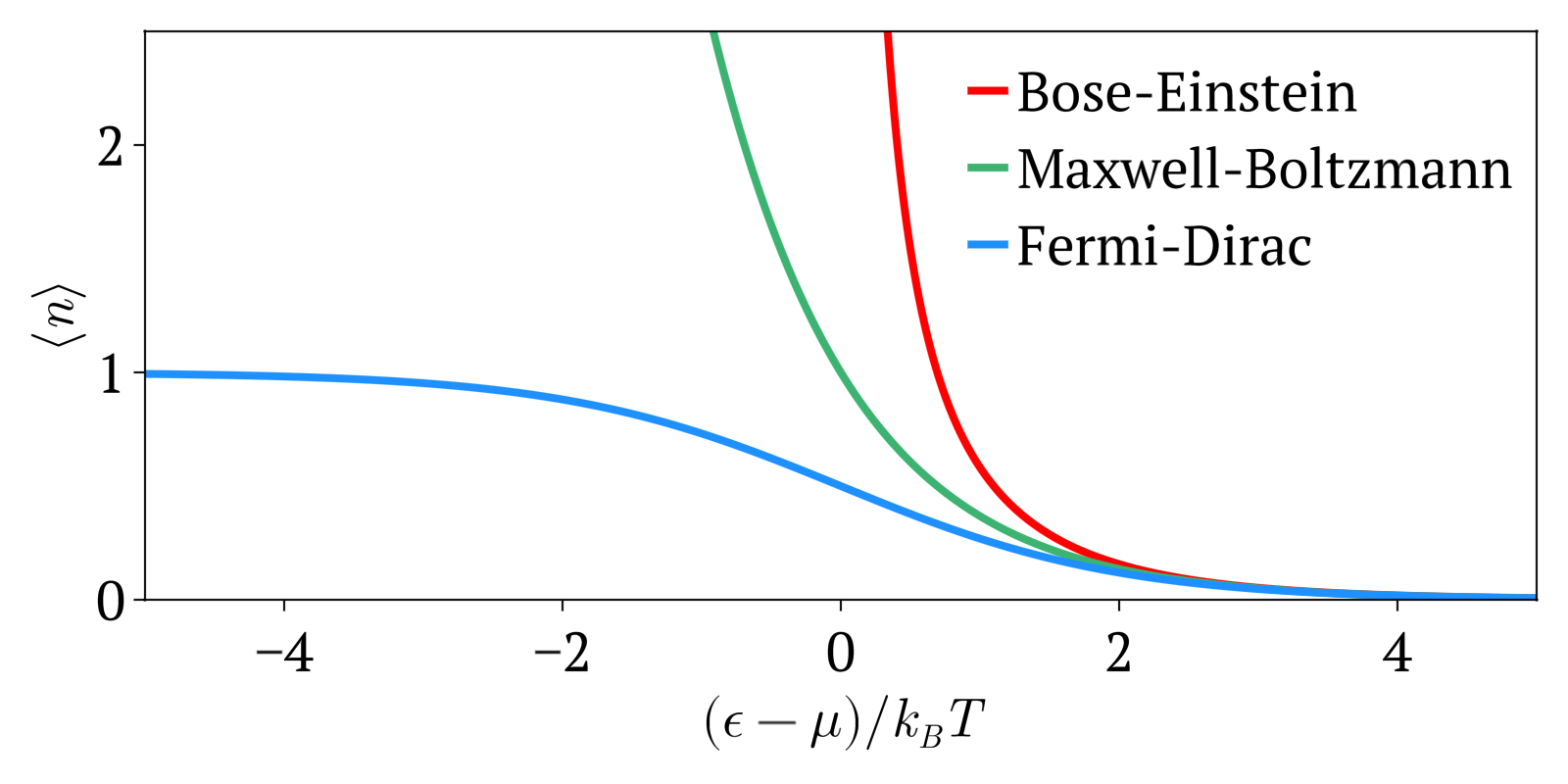
Maxwell–Boltzmann Statistics
In statistical mechanics, Maxwell–Boltzmann statistics describes the distribution of classical material particles over various energy states in thermal equilibrium. It is applicable when the temperature is high enough or the particle density is low enough to render quantum effects negligible. The expected number of particles with energy \varepsilon_i for Maxwell–Boltzmann statistics is : \langle N_i \rangle = \frac = \frac\,g_i e^, where: * \varepsilon_i is the energy of the ''i''th energy level, * \langle N_i \rangle is the average number of particles in the set of states with energy \varepsilon_i, * g_i is the degeneracy of energy level ''i'', that is, the number of states with energy \varepsilon_i which may nevertheless be distinguished from each other by some other means,For example, two simple point particles may have the same energy, but different momentum vectors. They may be distinguished from each other on this basis, and the degeneracy will be the number of po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Boltzmann Distribution
In statistical mechanics and mathematics, a Boltzmann distribution (also called Gibbs distribution Translated by J.B. Sykes and M.J. Kearsley. See section 28) is a probability distribution or probability measure that gives the probability that a system will be in a certain state as a function of that state's energy and the temperature of the system. The distribution is expressed in the form: :p_i \propto \exp\left(- \frac \right) where is the probability of the system being in state , is the exponential function, is the energy of that state, and a constant of the distribution is the product of the Boltzmann constant and thermodynamic temperature . The symbol \propto denotes proportionality (see for the proportionality constant). The term ''system'' here has a wide meaning; it can range from a collection of 'sufficient number' of atoms or a single atom to a macroscopic system such as a natural gas storage tank. Therefore, the Boltzmann distribution can be used to sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Variance
In probability theory and statistics, variance is the expected value of the squared deviation from the mean of a random variable. The standard deviation (SD) is obtained as the square root of the variance. Variance is a measure of dispersion, meaning it is a measure of how far a set of numbers is spread out from their average value. It is the second central moment of a distribution, and the covariance of the random variable with itself, and it is often represented by \sigma^2, s^2, \operatorname(X), V(X), or \mathbb(X). An advantage of variance as a measure of dispersion is that it is more amenable to algebraic manipulation than other measures of dispersion such as the expected absolute deviation; for example, the variance of a sum of uncorrelated random variables is equal to the sum of their variances. A disadvantage of the variance for practical applications is that, unlike the standard deviation, its units differ from the random variable, which is why the standard devi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Thermal Fluctuations
In statistical mechanics, thermal fluctuations are random deviations of an atomic system from its average state, that occur in a system at equilibrium.In statistical mechanics they are often simply referred to as fluctuations. All thermal fluctuations become larger and more frequent as the temperature increases, and likewise they decrease as temperature approaches absolute zero. Thermal fluctuations are a basic manifestation of the temperature of systems: A system at nonzero temperature does not stay in its equilibrium microscopic state, but instead randomly samples all possible states, with probabilities given by the Boltzmann distribution. Thermal fluctuations generally affect all the degrees of freedom of a system: There can be random vibrations (phonons), random rotations ( rotons), random electronic excitations, and so forth. Thermodynamic variables, such as pressure, temperature, or entropy, likewise undergo thermal fluctuations. For example, for a system that has an equ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
First Law Of Thermodynamics
The first law of thermodynamics is a formulation of the law of conservation of energy in the context of thermodynamic processes. For a thermodynamic process affecting a thermodynamic system without transfer of matter, the law distinguishes two principal forms of energy transfer, heat and thermodynamic work. The law also defines the internal energy of a system, an extensive property for taking account of the balance of heat transfer, thermodynamic work, and matter transfer, into and out of the system. Energy cannot be created or destroyed, but it can be transformed from one form to another. In an externally isolated system, with internal changes, the sum of all forms of energy is constant. An equivalent statement is that perpetual motion machines of the first kind are impossible; work done by a system on its surroundings requires that the system's internal energy be consumed, so that the amount of internal energy lost by that work must be resupplied as heat by an external e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Exact Differential
In multivariate calculus, a differential (infinitesimal), differential or differential form is said to be exact or perfect (''exact differential''), as contrasted with an inexact differential, if it is equal to the general differential dQ for some differentiable function Q in an Orthogonal coordinates, orthogonal coordinate system (hence Q is a multivariable function Dependent and independent variables#In pure mathematics, whose variables are independent, as they are always expected to be when treated in multivariable calculus). An exact differential is sometimes also called a ''total differential'', or a ''full differential'', or, in the study of differential geometry, it is termed an exact form. The integral of an exact differential over any integral path is Conservative vector field#Path independence, path-independent, and this fact is used to identify state functions in thermodynamics. Overview Definition Even if we work in three dimensions here, the definitions of exa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Finite Difference
A finite difference is a mathematical expression of the form . Finite differences (or the associated difference quotients) are often used as approximations of derivatives, such as in numerical differentiation. The difference operator, commonly denoted \Delta, is the operator (mathematics), operator that maps a function to the function \Delta[f] defined by \Delta[f](x) = f(x+1)-f(x). A difference equation is a functional equation that involves the finite difference operator in the same way as a differential equation involves derivatives. There are many similarities between difference equations and differential equations. Certain Recurrence relation#Relationship to difference equations narrowly defined, recurrence relations can be written as difference equations by replacing iteration notation with finite differences. In numerical analysis, finite differences are widely used for #Relation with derivatives, approximating derivatives, and the term "finite difference" is often used a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Potential
In thermodynamics, the chemical potential of a Chemical specie, species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species in a mixture is defined as the rate of change of Thermodynamic free energy, free energy of a thermodynamic system with respect to the change in the number of atoms or molecules of the species that are added to the system. Thus, it is the partial derivative of the free energy with respect to the amount of the species, all other species' concentrations in the mixture remaining constant. When both temperature and pressure are held constant, and the number of particles is expressed in moles, the chemical potential is the partial Molar concentration, molar Gibbs free energy. At chemical equilibrium or in phase equilibrium, the total sum of the product of chemical potentials and stoichiometric coefficients is zero, as the free en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |