|
Calcium-41
Calcium (20Ca) has 26 known isotopes, ranging from 35Ca to 60Ca. There are five stable isotopes (40Ca, 42Ca, 43Ca, 44Ca and 46Ca), plus one isotope (Calcium 48, 48Ca) with such a long half-life that for all practical purposes it can be considered stable. The most abundant isotope, 40Ca, as well as the rare 46Ca, are theoretically unstable on energetic grounds, but their decay has not been observed. Calcium also has a cosmogenic isotope, radioactive 41Ca, which has a half-life of 99,400 years. Unlike cosmogenic isotopes that are produced in the Earth's atmosphere, atmosphere, 41Ca is produced by neutron activation of 40Ca. Most of its production is in the upper metre of the soil column, where the cosmogenic neutron flux is still sufficiently strong. 41Ca has received much attention in stellar studies because it decays to 41K, a critical indicator of solar system anomalies. The most stable artificial radioisotopes are 45Ca with a half-life of 163 days and 47Ca with a half-life of 4. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cosmogenic Isotope
Cosmogenic nuclides (or cosmogenic isotopes) are rare nuclides (isotopes) created when a high-energy cosmic ray interacts with the atomic nucleus, nucleus of an ''in situ'' Solar System atom, causing nucleons (protons and neutrons) to be expelled from the atom (see cosmic ray spallation). These nuclides are produced within Earth materials such as rock (geology), rocks or soil, in Earth, Earth's Earth's atmosphere, atmosphere, and in extraterrestrial items such as meteoroids. By measuring cosmogenic nuclides, scientists are able to gain insight into a range of geology, geological and astronomy, astronomical processes. There are both radioactive isotope, radioactive and stable isotope, stable cosmogenic nuclides. Some of these radionuclides are tritium, carbon-14 and phosphorus-32. Certain light (low atomic number) primordial nuclides (isotopes of lithium, beryllium and boron) are thought to have been created not only during the Big Bang, but also (and perhaps primarily) to have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cosmogenic Nuclide
Cosmogenic nuclides (or cosmogenic isotopes) are rare nuclides (isotopes) created when a high-energy cosmic ray interacts with the nucleus of an '' in situ'' Solar System atom, causing nucleons (protons and neutrons) to be expelled from the atom (see cosmic ray spallation). These nuclides are produced within Earth materials such as rocks or soil, in Earth's atmosphere, and in extraterrestrial items such as meteoroids. By measuring cosmogenic nuclides, scientists are able to gain insight into a range of geological and astronomical processes. There are both radioactive and stable cosmogenic nuclides. Some of these radionuclides are tritium, carbon-14 and phosphorus-32. Certain light (low atomic number) primordial nuclides (isotopes of lithium, beryllium and boron) are thought to have been created not only during the Big Bang, but also (and perhaps primarily) to have been made after the Big Bang, but before the condensation of the Solar System, by the process of cosmic ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
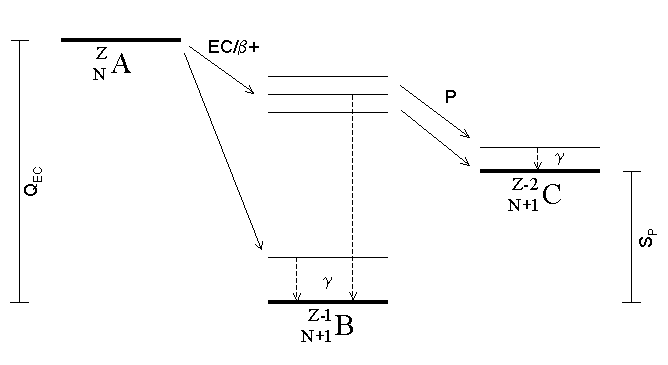
Proton Emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative—the proton is therefore unbound, and tunnels out of the nucleus in a finite time. Proton emission is not seen in naturally occurring isotopes; proton emitters can be produced via nuclear reactions, usually using linear particle accelerators. Although prompt (i.e. not beta-delayed) proton emission was observed from an isomer in cobalt-53 as early as 1969, no other proton-emitting states were found until 1981, when the proton radioactive ground states of lutetium-1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EPL (journal)
''EPL'' is a peer-reviewed scientific journal published by EDP Sciences, IOP Publishing and the Italian Physical Society on behalf of the European Physical Society and 17 other European physical societies. Prior to 1 January 2007 it was known as ''Europhysics Letters''. Scope ''EPL'' publishes original letters that communicates new results and findings that merit rapid publication in all areas of physics. ''EPL'' also publishes comments on letters previously published in the journal. History ''Europhysics Letters'' was founded in 1986 by the European Physical Society (EPS), Société Française de Physique (SFP) and its subsidiary EDP Sciences, the Società Italiana di Fisica (SIF) and the Institute of Physics (IOP). The new journal incorporated '' Lettere al Nuovo Cimento'' and '' Journal de Physique: Lettres'' and was published by EPS, EDP Sciences and SIF until 2006. ''EPL'' is now published under the scientific policy and control of the EPS by EDP Sciences, IOP Publishi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Half-life
A quantity is subject to exponential decay if it decreases at a rate proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and (lambda) is a positive rate called the exponential decay constant, disintegration constant, rate constant, or transformation constant: :\frac = -\lambda N. The solution to this equation (see derivation below) is: :N(t) = N_0 e^, where is the quantity at time , is the initial quantity, that is, the quantity at time . Measuring rates of decay Mean lifetime If the decaying quantity, ''N''(''t''), is the number of discrete elements in a certain set, it is possible to compute the average length of time that an element remains in the set. This is called the mean lifetime (or simply the lifetime), where the exponential time constant, \tau, relates to the decay rate constant, λ, in the following way: :\tau = \frac. The mean lifetime can be looked at as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Beta Decay
In nuclear physics, double beta decay is a type of radioactive decay in which two neutrons are simultaneously transformed into two protons, or vice versa, inside an atomic nucleus. As in single beta decay, this process allows the atom to move closer to the optimal ratio of protons and neutrons. As a result of this transformation, the nucleus emits two detectable beta particles, which are electrons or positrons. The literature distinguishes between two types of double beta decay: ''ordinary'' double beta decay and ''neutrinoless'' double beta decay. In ordinary double beta decay, which has been observed in several isotopes, two electrons and two electron antineutrinos are emitted from the decaying nucleus. In neutrinoless double beta decay, a hypothesized process that has never been observed, only electrons would be emitted. History The idea of double beta decay was first proposed by M. Goeppert-Mayer in 1935. In 1937, E. Majorana demonstrated that all results of beta decay t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primordial Nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the interstellar medium from which the solar system was formed, and were formed in, or after, the Big Bang, by nucleosynthesis in stars and supernovae followed by mass ejection, by cosmic ray spallation, and potentially from other processes. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present; 286 such nuclides are known. Stability All of the known 251 stable nuclides, plus another 35 nuclides that have half-lives long enough to have survived from the formation of the Earth, occur as primordial nuclides. These 35 primordial radionuclides represent isotopes of 28 separate elements. Cadmium, tellurium, xenon, neodymium, samarium, osm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium-48
Calcium-48 is a scarce isotope of calcium containing 20 protons and 28 neutrons. It makes up 0.187% of natural calcium by mole fraction. Although it is unusually neutron-rich for such a light nucleus, its beta decay is extremely hindered, and so the only radioactive decay pathway that it has been observed to undergo is the extremely rare process of double beta decay. Its half-life is about 6.4×1019 years, so for all practical purposes it can be treated as stable. One factor contributing to this unusual stability is that 20 and 28 are both magic numbers, making 48Ca a "doubly magic" nucleus. Since 48Ca is both practically stable and neutron-rich, it is a valuable starting material for the production of new nuclei in particle accelerators, both by fragmentation and by fusion reactions with other nuclei, for example in the discoveries of the heaviest five elements on the periodic table, from flerovium to oganesson. Heavier nuclei generally require a greater fraction of neutron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino. : : or when written as a nuclear reaction equation, ^_e + ^_p -> ^_n + ^_ ν_e Since this single emitted neutrino carries the entire decay energy, it has this single characteristic energy. Similarly, the momentum of the neutrino emission causes the daughter atom to recoil with a single characteristic momentum. The resulting daughter nuclide, if it is in an excited state, then transitions to its ground state. Usually, a gamma ray is emitted during this transition, but nuclear de-excitation may also take place by internal conversion. Following capture of an inner electron from the atom, an outer electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Electron Capture
Double electron capture is a decay mode of an atomic nucleus. For a nuclide (''A'', ''Z'') with a number of nucleons ''A'' and atomic number ''Z'', double electron capture is only possible if the mass of the nuclide (''A'', ''Z''−2) is lower. In this mode of decay, two of the orbital electrons are captured via the weak interaction by two protons in the nucleus, forming two neutrons (Two neutrinos are emitted in the process). Since the protons are changed to neutrons, the number of neutrons increases by two, while the number of protons ''Z'' decreases by two, and the atomic mass number ''A'' remains unchanged. As a result, by reducing the atomic number by two, double electron capture transforms the nuclide into a different element. Example: : Rarity In most cases this decay mode is masked by other, more probable modes involving fewer particles, such as single electron capture. When all other modes are “forbidden” (strongly suppressed) double electron capture becomes th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |