|
Cycloheptatriene
Cycloheptatriene (CHT) is an organic compound with the chemical formula, formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and a building block in organic synthesis. Cycloheptatriene is not aromatic, as reflected by the nonplanarity of the methylene bridge () with respect to the other atoms; however the related tropylium cation is, rendering the compound relatively suspectible to oxidation. Synthesis Albert Ladenburg first generated cycloheptatriene in 1881 by the decomposition of tropine. The structure was finally proven by the synthesis of Richard Willstätter in 1901. This synthesis started from cycloheptanone and established the seven membered ring structure of the compound. Cycloheptatriene can be obtained in the laboratory by photochemical reaction of benzene with d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buchner Ring Enlargement
The Buchner ring expansion is a two-step organic Carbon–carbon bond, C-C bond forming reaction used to access 7-membered Cyclic compound, rings. The first step involves formation of a carbene from ethyl diazoacetate, which cyclopropanation, cyclopropanates an aromatic ring. The Ring expansion reaction, ring expansion occurs in the second step, with an electrocyclic reaction opening the cyclopropane ring to form the 7-membered ring. and material science (fullerene derivatives). History The Buchner ring expansion reaction was first used in 1885 by Eduard Buchner and Theodor Curtius who prepared a carbene from ethyl diazoacetate for addition to benzene using both thermal and photochemical pathways in the synthesis of cycloheptatriene derivatives. The resulting product was a mixture of four isomeric carboxylic acids. Variations in the reaction arise from methods of carbene preparation. Advances in organometallic chemistry have resulted in increased selectivity of cycloheptatriene d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropylium Cation
The tropylium ion or cycloheptatrienyl cation is an aromatic species with a formula of 7H7sup>+. Its name derives from the molecule tropine from which cycloheptatriene (tropylidene) was first synthesized in 1881. Salts of the tropylium cation can be stable, even with nucleophiles of moderate strength e.g., tropylium tetrafluoroborate and tropylium bromide (''see below''). Its bromide and chloride salts can be made from cycloheptatriene and bromine or phosphorus pentachloride, respectively. It is a regular heptagonal, planar, cyclic ion. It has 6 π-electrons (4''n'' + 2, where ''n'' = 1), which fulfills Hückel's rule of aromaticity. It can coordinate as a ligand to metal atoms. The structure shown is a composite of seven resonance contributors in which each carbon atom carries part of the positive charge. History In 1891 G. Merling obtained a water-soluble bromine-containing compound from the reaction of cycloheptatriene and bromine. Unlike most al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sesquifulvalene
Sesquifulvalene or pentaheptafulvalene is a hydrocarbon in the fulvalene class with chemical formula C12H10. It is composed of linked cyclopentadiene and cycloheptatriene rings. Properties In the ground state, which is a singlet state, the central double bond is polarized, with a partial positive charge on the carbon atom of heptagonal ring and a partial negative charge on the carbon atom of pentagonal ring. This shift makes each ring have closer to 4''n''+2 π electrons, in keeping with the Hückel's pattern of aromatic stability. However, in the lowest quintet state, the central double bond is polarized with a partial negative charge on the carbon atom of heptagonal ring and a partial positive charge on the carbon atom of pentagonal ring due to Baird's rule. See also * Tropone * Biphenyl Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadiene
Cyclopentadiene is an organic compound with the chemical formula, formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimer (chemistry), dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction. This dimer can be retro-Diels–Alder reaction, restored by heating to give the monomer. The compound is mainly used for the production of cyclopentene and its derivatives. It is popularly used as a precursor to the cyclopentadienyl anion (Cp−), an important ligand in cyclopentadienyl complexes in organometallic chemistry. Production and reactions Cyclopentadiene production is usually not distinguished from dicyclopentadiene since they interconvert. They are obtained from coal tar (about 10–20 g/tonne, t) and by steam Cracking (chemistry), cracking of Petroleum naphtha, naphtha (about 14 kg/t) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azulene
Azulene is an aromatic organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. The compound is named after its colour, as "azul" is Spanish for blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that feature the azulene skeleton are found in nature as constituents of pigments in mushrooms, guaiac wood oil, and some marine invertebrates. Azulene has a long history, dating back to the 15th century as the azure-blue chromophore obtained by steam distillation of German chamomile. The chromophore was discovered in yarrow and wormwood and named in 1863 by Septimus Piesse. Its structure was first reported by Lavoslav Ružička, followed by its organic synthesis in 1937 by Placidus Plattner. Structure and bonding left, The blue color of the mushroom '' Lactarius indigo'' is due to the azulene derivative (7-isopropenyl-4-methylazulen-1-yl)methyl stearate. Azulene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heptalene
Heptalene is a polycyclic hydrocarbon with chemical formula , composed of two fused cycloheptatriene rings. It is an unstable, non-planar compound which is non-aromatic. The dianion, however, satisfies Hückel's rule In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was f ..., is thermally stable, and is planar. See also * Benzocyclooctatetraene References {{Hydrocarbon-stub Polycyclic nonaromatic hydrocarbons Bicyclic compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
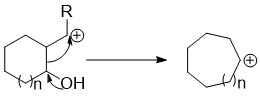
Ring Expansion Reaction
Ring expansion and ring contraction reactions expand or contract Ring (chemistry), rings, usually in organic chemistry. The term usually refers to reactions involve making and breaking C-C bonds, Diverse pathways lead to these kinds of reactions. Many of these reactions are primarily of theoretical or pedagoogical interest, but some are very useful. Ring expansions Rings can be expanded by attack of the ring onto an outside group already appended to the ring (a Migration (chemistry), migration/insertion), opening of a bicycle to a single larger ring, or coupling a ring closing with an expansion. These expansions can be further broken down by what type of atom they incorporate (a carbon or a heteroatom) into the expanded ring. Carbon insertion through migration to an exocyclic group These reactions have the general features of having an exocyclic leaving group on a carbon adjacent to the ring and an electron donating group on the ring capable of initiating a migration of an en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Diazoacetate
Ethyl diazoacetate (N=N=CHC(O)OC2H5) is a diazo compound and a reagent in organic chemistry. It was discovered by Theodor Curtius in 1883. The compound can be prepared by reaction of the ethyl ester of glycine with sodium nitrite and sodium acetate in water. : As a carbene precursor, it is used in the cyclopropanation of alkenes. Although the compound is hazardous, it is used in chemical industry as a precursor to trovafloxacin Trovafloxacin (sold as Trovan by Pfizer and Turvel by Laboratorios Almirall) is a broad spectrum antibiotic that inhibits the uncoiling of supercoiled DNA in various bacteria by blocking the activity of DNA gyrase and topoisomerase IV. It was .... Procedures for safe industrial handling have been published. Another location where EDA was used is in the production of BI-4752, a invented 5-HT2C agonist that is even better than lorcaserin. References {{Reflist Diazo compounds Reagents for organic chemistry Ethyl esters Conjugated ketones [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |