|
Cyanogen Glycosides
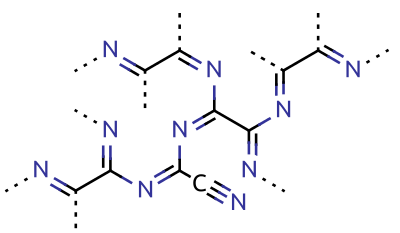
Cyanogen is the chemical compound with the formula . Its structure is . The simplest stable carbon nitride, it is a colorless and highly toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules are linear, and consist of two CN groups ‒ analogous to diatomic halogen molecules, such as Cl, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms, though other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide () (but see also ''Cyano radical''). When burned at increased pressure with oxygen, it is possible to get a blue tinted flame, the temperature of which is about 4800°C (a higher temperature is possible with ozone). It is as such regarded as the gas with the second highest temperature of burning (after dicyanoacetylene). Cyanogen is the anhydride of oxamide: : though oxamide is manufactured from cyanogen by hydrolysis: : Preparation Cya ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malononitrile
Malononitrile is an organic compound nitrile with the formula . It is a colorless or white solid, although aged samples appear yellow or even brown. It is a widely used building block in organic synthesis. Preparation and reactions It can be prepared by Dehydration reaction, dehydration of cyanoacetamide. This method is mainly practiced in China where environmental rules are lax. Most commonly malononitrile is produced by the gas-phase reaction of acetonitrile and cyanogen chloride: : About 20,000,000 kg are produced annually (2007). Important outlets include the synthesis of thiamine, the drug triamterene and minoxidil, and the dyes disperse Yellow 90 and disperse Blue 354. Malononitrile is relatively acidic, with a Acid dissociation constant, p''K''a of 11 in water. This allows it to be used in the Knoevenagel condensation, for example in the preparation of CS gas: Despite its relative obscurity, Malononitrile is very useful in several reactions, the prime example being a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidizing agent, oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and . However, the nature of fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Molecular Geometry
The linear molecular geometry describes the geometry around a central atom bonded to two other atoms (or ''ligands'') placed at a bond angle of 180°. Linear organic molecules, such as acetylene (), are often described by invoking sp orbital hybridization for their carbon centers. According to the VSEPR model (Valence Shell Electron Pair Repulsion model), linear geometry occurs at central atoms with two bonded atoms and zero or three lone pairs ( or ) in the AXE notation. Neutral molecules with linear geometry include beryllium fluoride () with two single bonds, carbon dioxide () with two double bonds, hydrogen cyanide () with one single and one triple bond. The most important linear molecule with more than three atoms is acetylene (), in which each of its carbon atoms is considered to be a central atom with a single bond to one hydrogen and a triple bond to the other carbon atom. Linear anions include azide () and thiocyanate (), and a linear cation is the nitronium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorganic molecules of the general forms ''Ps''–''Ps'' or ''Ps''–X (where ''Ps'' is a pseudohalogen group), such as cyanogen; pseudohalide anions, such as cyanide ion; inorganic acids, such as hydrogen cyanide; as ligands in coordination complexes, such as ferricyanide; and as functional groups in organic molecules, such as the nitrile group. Well-known pseudohalogen functional groups include cyanide, cyanate, thiocyanate, and azide. Common pseudohalogens and their nomenclature Many pseudohalogens are known by specialized common names according to where they occur in a compound. Well-known ones include (the true halogen chlorine is listed for comparison): is considered to be a pseudohalogen ion due to its disproportionation reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Odor
An odor (American English) or odour ( Commonwealth English; see spelling differences) is a smell or a scent caused by one or more volatilized chemical compounds generally found in low concentrations that humans and many animals can perceive via their olfactory system. While ''smell'' can refer to pleasant and unpleasant odors, the terms ''scent'', ''aroma'', and ''fragrance'' are usually reserved for pleasant-smelling odors and are frequently used in the food and cosmetic industry to describe floral scents or to refer to perfumes. Odor physiology Sense of smell The perception of odors, or sense of smell, is mediated by the olfactory nerve. The olfactory receptor (OR) cells are neurons present in the olfactory epithelium, which is a small patch of tissue at the back of the nasal cavity. There are millions of olfactory receptor neurons that act as sensory signaling cells. Each neuron has cilia in direct contact with the air. Odorous molecules bind to receptor pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pungency
Pungency ( ) is the taste of food commonly referred to as spiciness, hotness or heat, found in foods such as chili peppers. Highly pungent tastes may be experienced as unpleasant. The term piquancy ( ) is sometimes applied to foods with a lower degree of pungency that are "agreeably stimulating to the palate". Piquant ingredients include chili peppers, wasabi, horseradish and mustard. The primary substances responsible for pungent taste are capsaicin, piperine (in peppers) and allyl isothiocyanate (in radishes, mustard and wasabi). Terminology In colloquial speech, the term "pungency" can refer to any strong, sharp smell or flavor. However, in scientific speech, it refers specifically to the "hot" or "spicy" quality of chili peppers. It is the preferred term by scientists as it eliminates the ambiguity arising from use of "hot", which can also refer to temperature, and "spicy", which can also refer to spices. For instance, a pumpkin pie can be both hot (out of the oven) a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell ( cytotoxicity) or an organ such as the liver ( hepatotoxicity). Sometimes the word is more or less synonymous with poisoning in everyday usage. A central concept of toxicology is that the effects of a toxicant are dose-dependent; even water can lead to water intoxication when taken in too high a dose, whereas for even a very toxic substance such as snake venom there is a dose below which there is no detectable toxic effect. Toxicity is species-specific, making cross-species analysis problematic. Newer paradigms and metrics are evolving to bypass animal testing, while maintaining the concept of toxicity endpoints. Etymology In Ancient Greek medical literature, the adjective ''τοξικόν'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transparency And Translucency
In the field of optics, transparency (also called pellucidity or diaphaneity) is the physical property of allowing light to pass through the material without appreciable scattering of light. On a macroscopic scale (one in which the dimensions are much larger than the wavelengths of the photons in question), the photons can be said to follow Snell's law. Translucency (also called translucence or translucidity) is the physical property of allowing light to pass through the material (with or without scattering of light). It allows light to pass through but the light does not necessarily follow Snell's law on the macroscopic scale; the photons may be scattered at either of the two interfaces, or internally, where there is a change in the index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent materials appear clear, with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Nitride
Carbon nitrides are organic compounds consisting only of carbon and nitrogen atoms. Covalent network compounds These materials are organic semiconductors. Due to its hydrogen-bonding motifs and electron-rich properties, this carbon material is considered a potential candidate for material applications in carbon supplementation. * Beta carbon nitride - a solid with a formula , which is predicted to be harder than diamond. * Graphitic carbon nitride - , with important catalytic and sensor properties. * Dicyanodiazomethane , the only isomer studied experimentally * Tricyanamide - monomer (has never been prepared yet) * Dicyanocarbodiimide - another monomer (was detected in products of photolysis of triazido-s-triazine). * - a combined triazole and triazine framework. * MCN-12 () and MCN-13 (). Azafullerenes * Azafullerenes are a class of heterofullerenes in which the element substituting for carbon is nitrogen. Examples include (biazafullerenyl),Hummelen et al, "Is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |