|
Crazing
Crazing is a Yield (engineering), yielding mechanism in polymers characterized by the formation of a fine network of microvoids and fibrils. These structures (known as ''crazes'') typically appear as linear features and frequently precede brittle fracture. The fundamental difference between crazes and cracks is that crazes contain polymer fibrils (5-30 nm in diameter), constituting about 50% of their volume, whereas cracks do not. Unlike cracks, crazes can transmit load between their two faces through these fibrils. Crazes typically initiate when applied tensile stress causes microvoids to nucleate at points of high Stress (mechanics), stress concentration within the polymer, such as those created by scratches, flaws, cracks, dust particles, and molecular heterogeneities. Crazes grow normal to the principal (tensile) stress, they may extend up to centimeters in length and fractions of a millimeter in thickness if conditions prevent early failure and crack propagation. The ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compound (chemistry), compounds, produces unique physical property, physical properties including toughness, high rubber elasticity, elasticity, viscoelasticity, and a tendency to form Amorphous solid, amorphous and crystallization of polymers, semicrystalline structures rath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
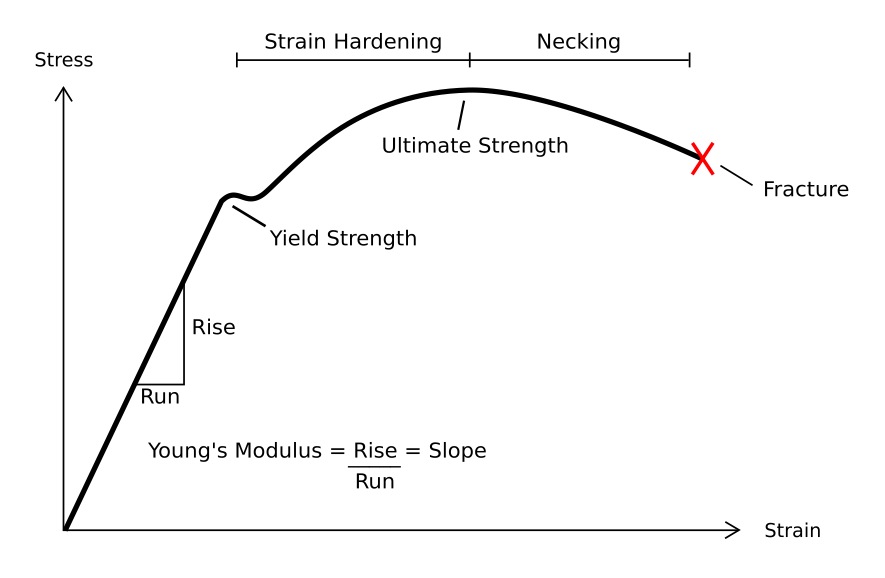
Plastic Deformation
In engineering, deformation (the change in size or shape of an object) may be ''elastic'' or ''plastic''. If the deformation is negligible, the object is said to be ''rigid''. Main concepts Occurrence of deformation in engineering applications is based on the following background concepts: * ''Displacements'' are any change in position of a point on the object, including whole-body translations and rotations ( rigid transformations). * ''Deformation'' are changes in the relative position between internals points on the object, excluding rigid transformations, causing the body to change shape or size. * ''Strain'' is the ''relative'' ''internal'' deformation, the dimensionless change in shape of an infinitesimal cube of material relative to a reference configuration. Mechanical strains are caused by mechanical stress, ''see stress-strain curve''. The relationship between stress and strain is generally linear and reversible up until the yield point and the deformation is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Craze Structure
Craze may refer to: * Craze, alternative name for fad * Craziness, alternative name for insanity * Crazing, a network of fine cracks People * Aaron Craze, English celebrity chef * DJ Craze (born 1977), Nicaraguan American DJ * Elizabeth Craze (born 1982), youngest ever heart transplant survivor at time of surgery (1984) * Galaxy Craze (born 1970), American actress * Michael Craze (1942–1998), British actor, brother of Peter Craze * Nathan Craze (born 1986), Welsh professional ice hockey player * Peter Craze (1946–2020), British actor, brother of Michael Craze * Richard Craze (1950–2006), British author * Romilly Craze (1892–1974), English architect * Sarah Craze (born 1948), British actress Events * Tulip craze in the 17th century in the Dutch Republic * Gin Craze in the first half of the 18th century in Britain * Pansy Craze in the late-1920s to mid-1930s in the United States * 1947 flying disc craze in the United States See also * Dance crazes, alternative name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluid
In physics, a fluid is a liquid, gas, or other material that may continuously motion, move and Deformation (physics), deform (''flow'') under an applied shear stress, or external force. They have zero shear modulus, or, in simpler terms, are Matter, substances which cannot resist any shear force applied to them. Although the term ''fluid'' generally includes both the liquid and gas phases, its definition varies among branches of science. Definitions of ''solid'' vary as well, and depending on field, some substances can have both fluid and solid properties. Non-Newtonian fluids like Silly Putty appear to behave similar to a solid when a sudden force is applied. Substances with a very high viscosity such as Pitch (resin), pitch appear to behave like a solid (see pitch drop experiment) as well. In particle physics, the concept is extended to include fluidic matters other than liquids or gases. A fluid in medicine or biology refers to any liquid constituent of the body (body fluid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be used: \rho = \frac, where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate this quantity is more specifically called specific weight. For a pure substance, the density is equal to its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium is the densest known element at standard conditions for temperature and pressure. To simplify comparisons of density across different systems of units, it is sometimes replaced by the dimensionless quantity "relative den ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Gerridae, water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to Cohesion (chemistry), cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmission Electron Microscopy
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a grid. An image is formed from the interaction of the electrons with the sample as the beam is transmitted through the specimen. The image is then magnified and focused onto an imaging device, such as a fluorescent screen, a layer of photographic film, or a detector such as a scintillator attached to a charge-coupled device or a direct electron detector. Transmission electron microscopes are capable of imaging at a significantly higher resolution than light microscopes, owing to the smaller de Broglie wavelength of electrons. This enables the instrument to capture fine detail—even as small as a single column of atoms, which is thousands of times smaller than a resolvable object seen in a light microscope. Transmission electron micr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meniscus (liquid)
In physics (particularly liquid statics), the meniscus (: menisci, ) is the curve in the upper surface of a liquid close to the surface of the container or another object, produced by surface tension. A concave meniscus occurs when the attraction between the particles of the liquid and the container ( adhesion) is more than half the attraction of the particles of the liquid to each other ( cohesion), causing the liquid to climb the walls of the container (see ). This occurs between water and glass. Water-based fluids like sap, honey, and milk also have a concave meniscus in glass or other wettable containers. Conversely, a convex meniscus occurs when the adhesion energy is less than half the cohesion energy. Convex menisci occur, for example, between mercury and glass in barometers and thermometers. In general, the shape of the surface of a liquid can be complex. For a sufficiently narrow tube with circular cross-section, the shape of the meniscus will approximate a sectio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Craze Growth Meniscus Instability
Craze may refer to: * Craze, alternative name for fad * Craziness, alternative name for insanity * Crazing, a network of fine cracks People * Aaron Craze, English celebrity chef * DJ Craze (born 1977), Nicaraguan American DJ * Elizabeth Craze (born 1982), youngest ever heart transplant survivor at time of surgery (1984) * Galaxy Craze (born 1970), American actress * Michael Craze (1942–1998), British actor, brother of Peter Craze * Nathan Craze (born 1986), Welsh professional ice hockey player * Peter Craze (1946–2020), British actor, brother of Michael Craze * Richard Craze (1950–2006), British author * Romilly Craze (1892–1974), English architect * Sarah Craze (born 1948), British actress Events * Tulip craze in the 17th century in the Dutch Republic * Gin Craze in the first half of the 18th century in Britain * Pansy Craze in the late-1920s to mid-1930s in the United States * 1947 flying disc craze in the United States See also * Dance crazes, alternative name ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rayleigh–Taylor Instability
The Rayleigh–Taylor instability, or RT instability (after Lord Rayleigh and G. I. Taylor), is an instability of an Interface (chemistry), interface between two fluids of different densities which occurs when the lighter fluid is pushing the heavier fluid. Philip Drazin, Drazin (2002) pp. 50–51. Examples include the behavior of water suspended above oil in the gravity of Earth, mushroom clouds like those from volcanic eruptions and atmospheric nuclear explosions, supernova explosions in which expanding core gas is accelerated into denser shell gas, merging binary quantum fluids in metastable configuration, instabilities in plasma fusion reactors and inertial confinement fusion. Concept Water suspended atop oil is an everyday example of Rayleigh–Taylor instability, and it may be scientific modeling, modeled by two completely plane-parallel layers of immiscible fluid, the denser fluid on top of the less dense one and both subject to the Earth's gravity. The Mechanical equili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |