|
Common-ion Effect
In chemistry, the common-ion effect refers to the decrease in solubility of an ionic precipitate by the addition to the solution of a soluble compound with an ion in common with the precipitate. This behaviour is a consequence of Le Chatelier's principle for the equilibrium reaction of the ionic association/ dissociation. The effect is commonly seen as an effect on the solubility of salts and other weak electrolytes. Adding an additional amount of one of the ions of the salt generally leads to increased precipitation of the salt, which reduces the concentration of both ions of the salt until the solubility equilibrium is reached. The effect is based on the fact that both the original salt and the other added chemical have one ion in common with each other. Examples of the common-ion effect Dissociation of hydrogen sulfide in presence of hydrochloric acid Hydrogen sulfide (H2S) is a weak electrolyte. It is partially ionized when in aqueous solution, therefore there exists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Etymology Because it was produced from halite, rock salt according to the methods of Johann Rudolph Glauber, hydrochloric acid was historically called by European alchemists ''spirits of salt'' or ''acidum salis'' (salt acid). Both names are still used, especially in other languages, such as , , , , , , , , , , (''ensan''), zh, 盐酸 (''yánsuān''), and (''yeomsan''). Gaseous HCl was called ''marine acid air''. The name ''muriatic acid'' has the same origin (''muriatic'' means "pertaining to brine or salt", hence ''muriate'' means hydrochloride), and this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ionic Strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation constant or the solubility of different salts. One of the main characteristics of a solution with dissolved ions is the ionic strength. Ionic strength can be molar (mol/L solution) or molal (mol/kg solvent) and to avoid confusion the units should be stated explicitly. The concept of ionic strength was first introduced by Lewis and Randall in 1921 while describing the activity coefficients of strong electrolytes. Quantifying ionic strength The molar ionic strength, ''I'', of a solution is a function of the concentration of ''all'' ions present in that solution. :I = \begin\frac\end\sum_^ c_i z_i^ where one half is because we are including both cations and anions, ''c''i is the molar concentration of ion i (M, mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Fatty Acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an Branched chain fatty acids, unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids (up to 70% by weight) in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important diet (nutrition), dietary sources of fuel for animals and important structural components for cell (biology), cells. History The concept of fatty acid (''acide gras'') was introduced in 1813 by Michel Eugène Chevreul, though he initially used some variant terms: ''graisse acide'' and ''acide huileux'' ("acid fat" and "oi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Soap
Soap is a salt (chemistry), salt of a fatty acid (sometimes other carboxylic acids) used for cleaning and lubricating products as well as other applications. In a domestic setting, soaps, specifically "toilet soaps", are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are used as thickeners, components of some lubricants, emulsifiers, and catalysts. Soaps are often produced by mixing fats and oils with a Base (chemistry), base. Humans have used soap for millennia; evidence exists for the production of soap-like materials in ancient Babylon around 2800 BC. Types Toilet soaps In a domestic setting, "soap" usually refers to what is technically called a toilet soap, used for household and personal cleaning. Toilet soaps are salts of fatty acids with the general formula (Carboxylate ion, RCO2−)M+, where M is Sodium, Na (sodium) or Potassium, K (potassium). When used for cleaning, soap solubilizes particles and g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
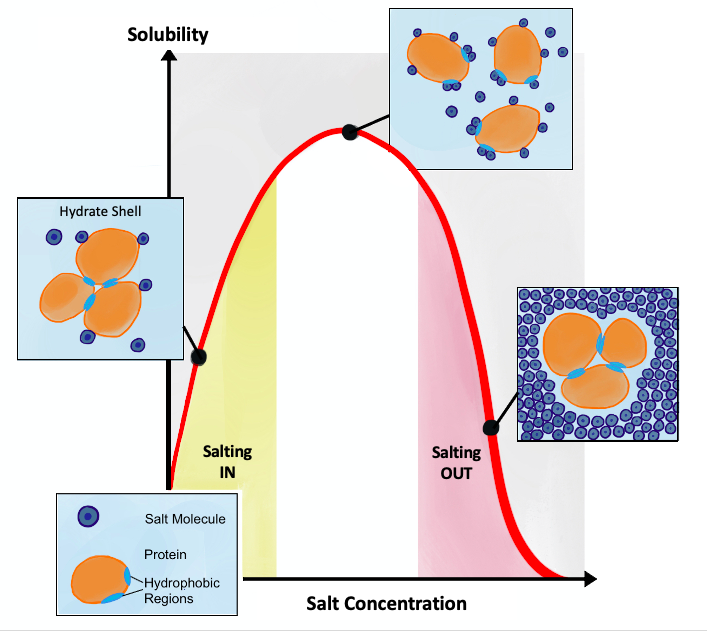
Salting-out
Salting out (also known as salt-induced precipitation, salt fractionation, anti-solvent crystallization, precipitation crystallization, or drowning out) is a purification technique that utilizes the reduced solubility of certain molecules in a solution of very high ionic strength. Salting out is typically used to precipitate large biomolecules, such as proteins or DNA. Because the salt concentration needed for a given protein to precipitate out of the solution differs from protein to protein, a specific salt concentration can be used to precipitate a target protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed. Principle Salt compounds dissociate in aqueous solutions. This property is exploited in the process of salting out. When the salt concentration is increased, some of the water molecules are attracted by the salt ions, which decreases the number of water molecules available to interact with the ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Toothpaste
Toothpaste is a paste or gel dentifrice that is used with a toothbrush to clean and maintain the aesthetics of Human tooth, teeth. Toothpaste is used to promote oral hygiene: it is an abrasive that aids in removing dental plaque and food from the teeth, assists in suppressing halitosis, and delivers active ingredients (most commonly fluoride) to help prevent tooth decay (dental caries) and gum disease (gingivitis).American Dental Association Description of Toothpaste Due to variations in composition and fluoride content, not all toothpastes are equally effective in maintaining oral health. The decline of tooth decay during the 20th century has been attributed to the introduction and regular use of fluoride-containing toothpastes worldwide. Large amounts of swallowed toothpaste can be poisonous. Common colors for toothpaste include white (sometimes with colored stripes or green tint) and blue. History Early toothpastes Since 5000 BCE, the Egyptians made a tooth powder, which cons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Calcium Carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues. Chemistry Calcium carbonate shares the typical properties of other carbonates. Notably, it: *reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water: : *releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above 840 °C in the case of ), t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Sodium Carbonate
Sodium carbonate (also known as washing soda, soda ash, sal soda, and soda crystals) is the inorganic compound with the formula and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the chloralkali process. Hydrates Sodium carbonate is obtained as three hydrates and as the anhydrous salt: * sodium carbonate decahydrate ( natron), Na2CO3·10H2O, which readily effloresces to form the monohydrate. * sodium carbonate heptahydrate (not known in mineral form), Na2CO3·7H2O. * sodium carbonate monohydrate ( thermonatr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Water Treatment
Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, including being safely returned to the environment. Water treatment removes contaminants and undesirable components, or reduces their concentration so that the water becomes fit for its desired end-use. This treatment is crucial to human health and allows humans to benefit from both drinking and irrigation use. Types Drinking water treatment Water contamination is primarily caused by the discharge of untreated wastewater from enterprises. The effluent from various enterprises, which contains varying levels of contaminants, is dumped into rivers or other water resources. The wastewater may have a high proportion of organic and inorganic contaminants at the initial discharge. Industries generate wastewater as a result of fabrica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hard Water
Hard water is water that has a high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates. Drinking hard water may have moderate health benefits. It can pose critical problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling towers, and other equipment that handles water. In domestic settings, hard water is often indicated by a lack of foam formation when soap is agitated in water, and by the formation of limescale in kettles and water heaters. Wherever water hardness is a concern, water softening is commonly used to reduce hard water's adverse effects. Origins Natural rainwater, snow and other forms of precipitation typically have low concentrations of divalent cations such as calcium and magnesium. They may have small concentrations of ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Aquifers
An aquifer is an underground layer of water-bearing material, consisting of permeability (Earth sciences), permeable or fractured rock, or of unconsolidated materials (gravel, sand, or silt). Aquifers vary greatly in their characteristics. The study of water flow in aquifers and the characterization of aquifers is called ''hydrogeology''. Related concepts include aquitard, a bed (geology), bed of low permeability along an aquifer, and aquiclude (or ''aquifuge''), a solid and impermeable region underlying or overlying an aquifer, the pressure of which could lead to the formation of a confined aquifer. Aquifers can be classified as saturated versus unsaturated; aquifers versus aquitards; confined versus unconfined; isotropic versus anisotropic; porous, karst, or fractured; and transboundary aquifer. Groundwater from aquifers can be sustainably harvested by humans through the use of qanats leading to a well. This groundwater is a major source of fresh water for many regions, althoug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |