|
Chlorine-37
Chlorine-37 (), is one of the stable isotopes of chlorine, the other being chlorine-35 (). Its nucleus contains 17 protons and 20 neutrons for a total of 37 nucleons. Chlorine-37 accounts for 24.23% of natural chlorine, chlorine-35 accounting for 75.77%, giving chlorine atoms in bulk an apparent atomic weight of . Remarkably, solar neutrinos were discovered by an experiment ( Homestake Experiment) using a radiochemical method based on chlorine-37 transmutation. Neutrino detection One of the historically important radiochemical methods of solar neutrino detection is based on inverse electron capture triggered by the absorption of an electron neutrino. Chlorine-37 transmutes into argon-37 via the reaction : + → + . Argon-37 then decays via electron capture (half-life 35 d) into chlorine-37 via the reaction : + → + . These last reactions involve Auger electrons of specific energies. The detection of these electrons confirms that a neutrino event occurred. Det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Chlorine
Chlorine (17Cl) has 25 isotopes, ranging from 28Cl to 52Cl, and two isomers, 34mCl and 38mCl. There are two stable isotopes, 35Cl (75.8%) and 37Cl (24.2%), giving chlorine a standard atomic weight of 35.45. The longest-lived radioactive isotope is 36Cl, which has a half-life of 301,000 years. All other isotopes have half-lives under 1 hour, many less than one second. The shortest-lived are proton-unbound 29Cl and 30Cl, with half-lives less than 10 picoseconds and 30 nanoseconds, respectively; the half-life of 28Cl is unknown. List of isotopes , -id=Chlorine-28 , 28Cl , style="text-align:right" , 17 , style="text-align:right" , 11 , 28.03035(54)# , , p , 27S , 1+# , , , -id=Chlorine-29 , 29Cl , style="text-align:right" , 17 , style="text-align:right" , 12 , 29.01505(20)# , 5.4(19) zs , p , 28S , (1/2+) , , , -id=Chlorine-30 , 30Cl , style="text-align:right" , 17 , style="text-align:right" , 13 , 30.005018(26) , 92%) , 44Ar , rowspan=2, (2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Isotope
Stable nuclides are Isotope, isotopes of a chemical element whose Nucleon, nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The Atomic nucleus, nuclei of such isotopes are not radioactive and unlike radionuclides do not spontaneously undergo radioactive decay. When these nuclides are referred to in relation to specific elements they are usually called that element's stable isotopes. The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been shown to decay using current equipment. Of these 80 elements, 26 have only one stable isotope and are called monoisotopic element, monoisotopic. The other 56 have more than one stable isotope. Tin has ten stable isotopes, the largest number of any element. Definition of stability, and naturally occurring nuclides Most naturally occurring nuclides are stable (about 251; see list at the end of this article), and about 35 more (tot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the Department_of_Physics_and_Astronomy,_University_of_Manchester , University of Manchester based on the 1909 Geiger–Marsden experiments, Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a non-flammable, dense, colourless liquid with a "sweet" chloroform-like odour that can be detected at low levels. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, an anthelmintic and a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal. Properties In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedron, tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetric geometry, CCl4 is non-polar. methane, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopic Tracer
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through chemical reaction, metabolic pathway, or a Cell (biology), biological cell. The reactant is 'labeled' by replacing one or more specific atoms with their isotopes. The reactant is then allowed to undergo the reaction. The position of the isotopes in the product (chemistry), products is measured to determine what sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling. In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
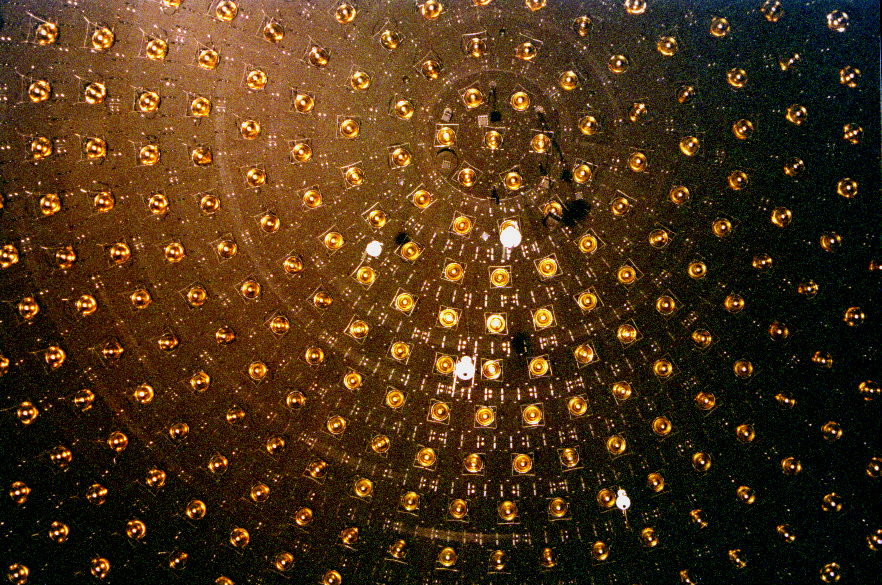
Neutrino Detection
A neutrino detector is a physics apparatus which is designed to study neutrinos. Because neutrinos only weakly interact with other particles of matter, neutrino detectors must be very large to detect a significant number of neutrinos. Neutrino detectors are often built underground, to isolate the detector from cosmic rays and other background radiation. The field of neutrino astronomy is still very much in its infancy – the only confirmed extraterrestrial sources are the Sun and the supernova 1987A in the nearby Large Magellanic Cloud. Another likely source (three standard deviations) is the blazar TXS 0506+056 about 3.7 billion light years away. Neutrino observatories will "give astronomers fresh eyes with which to study the universe". Various detection methods have been used. Super Kamiokande is a large volume of water surrounded by phototubes that watch for the Cherenkov radiation emitted when an incoming neutrino creates an electron or muon in the water. The Sudbu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in what is called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron emission to be energeticall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parts Per Thousand
In science and engineering, the parts-per notation is a set of pseudo-units to describe the small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement. Commonly used are * parts-per-million - ppm, * parts-per-billion - ppb, * parts-per-trillion - ppt, * parts-per-quadrillion - ppq, This notation is not part of the International System of Units - SI system and its meaning is ambiguous. Applications Parts-per notation is often used describing dilute solutions in chemistry, for instance, the relative abundance of dissolved minerals or pollutants in water. The quantity "1 ppm" can be used for a mass fraction if a water-borne pollutant is present at one-millionth of a gram per gram of sample solution. When working with aqueous solutions, it is common to assume that the density of water is 1.00 g/mL. Therefore, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organochlorine Compound
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds that contain one or more carbon–chlorine bonds. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Organochlorides such as trichloroethylene, tetrachloroethylene, dichloromethane and chloroform are commonly used as solvents and are referred to as "chlorinated solvents". Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. They have highe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Reference Material
Standard may refer to: Symbols * Colours, standards and guidons, kinds of military signs * Standard (emblem), a type of a large symbol or emblem used for identification Norms, conventions or requirements * Standard (metrology), an object that bears a defined relationship to a unit of measure used for calibration of measuring devices * Standard (timber unit), an obsolete measure of timber used in trade * Breed standard (also called bench standard), in animal fancy and animal husbandry * BioCompute Standard, a standard for next generation sequencing * ''De facto'' standard, product or system with market dominance * Gold standard, a monetary system based on gold; also used metaphorically for the best of several options, against which the others are measured * Internet Standard, a specification ratified as an open standard by the Internet Engineering Task Force * Learning standards, standards applied to education content * Standard displacement, a naval term describing the weig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NIST
The National Institute of Standards and Technology (NIST) is an agency of the United States Department of Commerce whose mission is to promote American innovation and industrial competitiveness. NIST's activities are organized into physical science laboratory programs that include nanoscale science and technology, engineering, information technology, neutron research, material measurement, and physical measurement. From 1901 to 1988, the agency was named the National Bureau of Standards. History Background The Articles of Confederation, ratified by the colonies in 1781, provided: The United States in Congress assembled shall also have the sole and exclusive right and power of regulating the alloy and value of coin struck by their own authority, or by that of the respective states—fixing the standards of weights and measures throughout the United States. Article 1, section 8, of the Constitution of the United States, ratified in 1789, granted these powers to the new Co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World Scientific
World Scientific Publishing is an academic publisher of scientific, technical, and medical books and journals headquartered in Singapore. The company was founded in 1981. It publishes about 600 books annually, with more than 170 journals in various fields. In 1995, World Scientific co-founded the London-based Imperial College Press together with the Imperial College of Science, Technology and Medicine. Company structure The company head office is in Singapore. The Chairman and Editor-in-Chief is Dr Phua Kok Khoo, while the Managing Director is Doreen Liu. The company was co-founded by them in 1981. Imperial College Press In 1995 the company co-founded Imperial College Press, specializing in engineering, medicine and information technology Information technology (IT) is a set of related fields within information and communications technology (ICT), that encompass computer systems, software, programming languages, data processing, data and information processing, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |