|
Cenegermin
Cenegermin, sold under the brand name Oxervate, also known as recombinant human nerve growth factor, is a recombinant form of human nerve growth factor. Cenegermin is a peripherally selective agonist An agonist is a chemical that activates a Receptor (biochemistry), receptor to produce a biological response. Receptors are Cell (biology), cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an R ... of the tropomyosin receptor kinase A (TrkA) and low-affinity nerve growth factor receptor (p75NTR). The most common side effects include eye pain and inflammation, increased lacrimation (watery eyes), pain in the eyelid and sensation of a foreign body in the eye. It was approved for medical use in the European Union in July 2017, and in the United States in 2018. The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. Medical uses Cenegermin is indicated for the treatment of neurotrophic kerati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nerve Growth Factor
Nerve growth factor (NGF) is a neurotrophic factor and neuropeptide primarily involved in the regulation of growth, maintenance, proliferation, and survival of certain target neurons. It is perhaps the prototypical growth factor, in that it was one of the first to be described. Since it was first isolated by Nobel laureates Rita Levi-Montalcini and Stanley Cohen in 1954, numerous biological processes involving NGF have been identified, two of them being the survival of pancreatic beta cells and the regulation of the immune system. Structure NGF is initially in a 7S, 130- kDa complex of 3 proteins – Alpha-NGF, Beta-NGF, and Gamma-NGF (2:1:2 ratio) when expressed. This form of NGF is also referred to as proNGF (NGF precursor). The gamma subunit of this complex acts as a serine protease, and cleaves the N-terminal of the beta subunit, thereby activating the protein into functional NGF. The term ''nerve growth factor'' usually refers to the 2.5S, 26-kDa beta subunit of the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ophthalmic Drug Administration
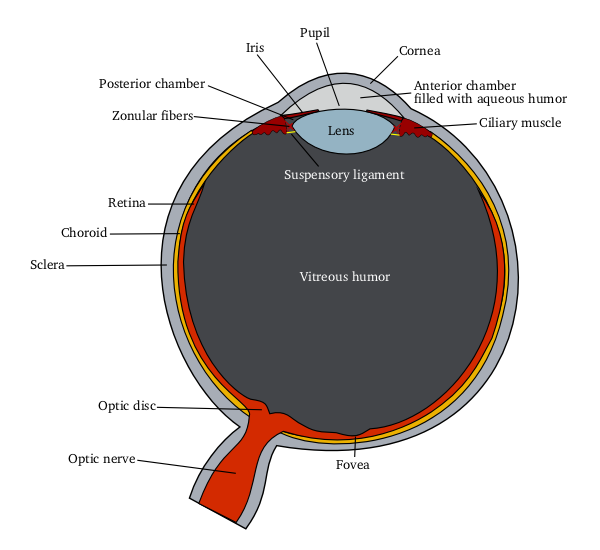
Ophthalmic drug administration is the administration of a drug to the eyes, most typically as an eye drop formulation. Topical formulations are used to combat a multitude of diseased states of the eye. These states may include bacterial infections, eye injury, glaucoma, and dry eye. However, there are many challenges associated with topical delivery of drugs to the cornea of the eye. Eye drop formulations Two of the largest challenges faced when using topicals to treat pathological states of the eye include Adherence (medicine), patient compliance and ineffective absorbance of drugs into the cornea due to short contact times, solution drainage, tears turnover, and dilution or lacrimation. In fact, researchers in this field of drug delivery agree that less than 7% of drugs delivered to the eye reach and penetrate the corneal barrier, therefore, increasing the frequency of dosing used for topicals. This is one of the fundamental problem associated with using topicals to deliver dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C). However, the agency also enforces other laws, notably Section 361 of the Public Health Service Act as well as associated regulations. Much of this regulatory-enforcement work is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ophthalmology Drugs
Ophthalmology (, ) is the branch of medicine that deals with the diagnosis, treatment, and surgery of eye diseases and disorders. An ophthalmologist is a physician who undergoes subspecialty training in medical and surgical eye care. Following a medical degree, a doctor specialising in ophthalmology must pursue additional postgraduate residency training specific to that field. In the United States, following graduation from medical school, one must complete a four-year residency in ophthalmology to become an ophthalmologist. Following residency, additional specialty training (or fellowship) may be sought in a particular aspect of eye pathology. Ophthalmologists prescribe medications to treat ailments, such as eye diseases, implement laser therapy, and perform surgery when needed. Ophthalmologists provide both primary and specialty eye care—medical and surgical. Most ophthalmologists participate in academic research on eye diseases at some point in their training and many inclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurotrophic Factors
Neurotrophic factors (NTFs) are a family of biomolecules – nearly all of which are peptides or small proteins – that support the growth, survival, and cell differentiation, differentiation of both developing and mature neurons. Most NTFs exert their trophic effects on neurons by signaling through tyrosine kinases, usually a receptor tyrosine kinase. In the mature nervous system, they promote neuronal survival, induce synaptic plasticity, and modulate the formation of long-term memories. Neurotrophic factors also promote the initial growth and development of neurons in the central nervous system and peripheral nervous system, and they are capable of regrowing damaged neurons in test tubes and animal models. Some neurotrophic factors are also released by the target tissue in order to axon guidance, guide the growth of developing axons. Most neurotrophic factors belong to one of three families: (1) neurotrophins, (2) glial cell-line derived neurotrophic factor family liga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' ( )Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. is a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus '' Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are part of the normal microbiota of the gut, where they constitute about 0.1%, along with other facultative anaerobes. These bacteria are mostly harmless or even beneficial to humans. For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by harmful pathogenic bacteria. These mutually beneficial relationships between ''E. coli'' and humans are a type of mutualistic biological relationship—where both the humans and the ''E. coli'' are benefitting each other. ''E. coli'' is expelled into the environment within fecal matter. The bacterium grows massi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indicated
In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedure or medication. An indication can commonly be confused with the term diagnosis. A diagnosis is the assessment that a particular medical condition is present while an indication is a reason for use. The opposite of an indication is a contraindication, a reason to withhold a certain medical treatment because the risks of treatment clearly outweigh the benefits. In the United States, indications for prescription drugs are approved by the FDA. Indications are included in the Indications and Usage section of the Prescribing Information. The primary role of this section of labeling is to enable health care practitioners to readily identify appropriate therapies for patients by clearly communicating the drug's approved indication(s). The Indications and Usage section states the disease or condition, or manifestation or symptoms thereof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First-in-class Medication
A first-in-class medication is a prototype drug that uses a "new and unique mechanism of action" to treat a particular medical condition. While the Food and Drug Administration's Center for Drug Evaluation and Research tracks first-in-class medications and reports on them annually, first-in-class is not considered a regulatory category. Although many first-in-class medications qualify as breakthrough therapies, Regenerative Medicine Advanced Therapies and/or orphan drugs, first-in-class status itself has no regulatory effect. Examples Controversy Safety By definition, a first-in-class drug does not have the safety evidence from analogous products that not-first-in-class drugs would have. However, a study investigating recalls and warnings in relation to first-in-class drugs approved between 1997 and 2012 by Health Canada has found that first-in-class drugs actually have a more favourable benefit-to-harm ratio. Economics First-in-class drugs are often seen as commerc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Therapeutic Goods Administration
The Therapeutic Goods Administration (TGA) is the medicine and therapeutic regulatory agency of the Australian Government. As part of the Department of Health, Disability and Ageing, the TGA regulates the safety, quality, efficacy and advertising in Australia of therapeutic goods (which comprise medicines, medical devices, biologicals and certain other therapeutic goods). Therapeutic goods include goods that are represented to have a therapeutic effect, are included in a class of goods the sole or principal use of which is (or ordinarily is) a therapeutic use, or are otherwise determined to be a therapeutic good through a legislative instrument under the ''Therapeutic Goods Act 1989.'' Goods that are therapeutic goods must be entered on the Australian Register of Therapeutic Goods (ARTG), or otherwise be the subject of an exemption, approval or authority by the TGA under the ''Therapeutic Goods Act 1989'', ''Therapeutic Goods Regulations 1990'' or ''Therapeutic Goods (Medical Dev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low-affinity Nerve Growth Factor Receptor
The p75 neurotrophin receptor (p75NTR) was first identified in 1973 as the low-affinity nerve growth factor receptor (LNGFR) before discovery that p75NTR bound other neurotrophins equally well as nerve growth factor. p75NTR is a neurotrophic factor receptor. Neurotrophic factor receptors bind Neurotrophins including Nerve growth factor, Neurotrophin-3, Brain-derived neurotrophic factor, and Neurotrophin-4. All neurotrophins bind to p75NTR. This also includes the immature pro-neurotrophin forms. Neurotrophic factor receptors, including p75NTR, are responsible for ensuring a proper density to target ratio of developing neurons, refining broader maps in development into precise connections. p75NTR is involved in pathways that promote neuronal survival and neuronal death. Receptor family p75NTR is a member of the TNF receptor superfamily, tumor necrosis factor receptor superfamily. p75NTR/LNGFR was the first member of this large family of receptors to be characterized, that now contai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |